A UNIVERSE FULL OF HOVERING BUTTS AND WHY IT IS IMPOSSIBLE TO KISS SOMEONE
Are you sure they kissed? Maybe they did not. I understand you saw them kiss right before your eyes. But how do I know they were really where you saw them? How do I know you were even there looking at them? We, my friend, are stuck in a deceptive universe.
Ever since we started leaving footprints on the moist earth, we have questioned our very existence, why are we here, where are we headed, and whether the universe had been fine-tuned by some higher entity so that it exists just like the way it is. Seeing from our vantage point and in absence of the slightest hint of evidence n favour of extraterrestrial intelligence or the aforesaid higher entity, we seem to be the only ones asking the big questions. It is inherently human of us to search for the absolute truth and not just a scraped version providentially restricted by the limit of our senses. With thousands of years of accumulated knowledge behind our backs, giant particle accelerators and equally intimidating gravity wave detectors, neutrino detectors and a 10 billion dollar space telescope at our fingertips, we envision a future wherein the being of the whole universe is to be explained by a fundamental set of physical laws and mathematical equations. But there remains one problem.
We inhabit a deceptive, misleading universe, where the long-believed notion that space and time are absolute appears to be relative. Space becomes bendy and stretchy and warps and folds. On the other hand, time, which is synonymous with the ticking of clocks has no definite meaning as the passing of time or the interval separating two events, in all essence, depends on the state of the clock which could be undergoing accelerated motion or approaching a region of intense gravitational potential. From Einstein's twin theories of relativity, we conclude that the fixed, unchanging notion of reality has become indefinite. As if the situation is not already worse, there came quantum mechanics, which showed us that whatever we see, hear or feel is nothing but walls upon walls of illusory phenomena originating from the fundamental laws that govern the universe. No matter how much we try to emphasize that we are ''really'' holding a cup of coffee, quantum mechanics says that there could be no us, just like there could be no cup of coffee to begin with.
In order to learn more about the problem in question and the purpose of this article, we need to imagine a young couple walking hand-in-hand. Being new in love, it is time for their first passionate kiss. They steadily lean in, their lips crash, and as they both push their way into each other, they are locked in a mutual embrace. For those who have never been in love, all they need to do is sit on a chair and look at their own selves. There is one more thing; we need to make ourselves a cup of coffee and hold it exactly like the photograph below.
 |
| You sure you are holding it right? Think my friend, think again. Remember, this is a deceptive universe. Image Credits: Photo by Pixnio |
All these appear to be absolutely, totally, unequivocally and unquestionably real, as in ''really'' real. We do these things we kiss, we like to throw our butts on that squishy sofa the moment we return from a wearisome job, and maybe, at this very moment, some of us are holding a cup of coffee. However, we are living under the misapprehension that nothing out-of-the-ordinary is happening here, even though there is more to it than meets the eye.
This failure to grok the significance of the little things in our lives comes from the fact that we get to experience a very limited fraction of the totality of things out there, starting from entities of the order of 10⁻³⁵ m (the Planck length) to 93 billion light-years stretch across the diameter of the observable universe. Both numbers on either side outrank our human perspectives by stupendously large orders of magnitudes. We should bear in mind that our human senses have evolved concerning our local neighbourhood. We never had the necessity to see beyond the horizon or detect wavelengths other than what we call the visible spectrum or taste the very atoms that make an apple sweet. This somewhat explains why our eyes are exactly the way it is. And so are our other senses; touch, hearing, taste, and smell. There has been and always will be a limitation to how much we can perceive of the world around us and the universe. It is only through sheer intellectual discernment and, in some cases, through the hardest mathematical expressions and consistent physical laws could we get a taste of the absolute reality, a reality hidden behind our familiar experiences. Then only we will realise that the couple never actually kissed, and no single one of us is sitting on a chair. Instead, our beautiful butts are hovering in thin air while the chair itself floats above the floor.
 |
| They kissed. They kissed? Are they even there? Or am I even here? Image Credits: Photo by pixabay |
When it comes to the world of atoms, we are taught that all material objects, starting from stars, galaxies, planets, moons, car keys, coffee mugs, including our very own bodies and that pet cat curled up on our laps are made up of many billions upon billions of atoms (see The atomic theory of matter). For an average 70 kg adult male, there are somewhere around 7×10²⁷ (i.e., 7 octillion) atoms of 41 major elements, including some other atomic species in trace amounts which are responsible for the proper functioning of our bodies⁽¹⁾. Initially, we learn to picture an atom very much like a miniaturised version of the solar system. Accordingly, an atom comprises a central region, called the nucleus (in place of the Sun) which is composed of positively charged species called protons and a neutral species called neutrons. The nucleus occupies a very small fraction of the total atomic volume. The nucleus is then surrounded by negatively charged electrons which revolve around the former in fixed orbits (analogous to the planets). Unless an atom is radioactive, it contains an equal number of protons, electrons and neutrons which behave much like solid billiard balls. Additionally, an atom as a whole is mostly empty space, i.e., almost 99% of the total occupied volume of an atom is simply nothing at all. The pair of schematic diagrams below summarises the classical picture of an atom.
 |
| Schematic diagram of the solar system Image Credits: Pixabay |
 |
| The Bohr Atom Model Image Credits: Pixabay |
But as the classical picture grapples with numerous inconsistencies, to explain the behaviour of atoms, we need to apply the laws of quantum mechanics, wherefore, our idea of the said atom changes drastically. Quantum mechanics states that an atom as a whole is nothing remotely like a solid object of our day to day lives. It is not a billiard ball, something which we can see or touch, and what we simply call empty space is actually not empty but filled with radiation and virtual particles. However, in this present article, we have to skip much of quantum mechanics, for the sake of keeping our discussion simple and comprehensible to the laypeople.
For now, we require a very little amount of quantum mechanics which is, unfortunately, the only standard way of explaining what an electron might look like. Quantum mechanically, an electron is ascribed to a complex-valued wavefunction, which is a completely abstract mathematical description of an electron's existence (mind the term). As it is near enough impossible to describe a wavefunction using ordinary English language, we can picture an atom as follows. Unlike the classical solar-system model of an atom, a quantum mechanical atom exists as a fuzzy cloud of probability amplitudes. The classical picture of the solid billiard ball-like electron (see The Rutherford Atom Model) is replaced by a probability wave which means that there is only a chance of finding an electron near the vicinity of an atomic nuclei. Quantum mechanics is so abstract a theory and works on level that is completely inaccessible to us, that at some point we come to the conclusion that electrons do not actually exist in the truest sense of the word but rather our attempt to observe an electron causes it to exist. For the sake of brevity, this is where we must depart from the quantum picture and instead, take a good look at the following diagram.
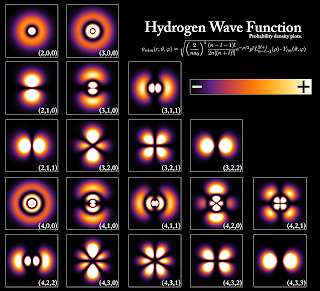 |
| This is what a hydrogen atom looks like according to quantum mechanics. Image Credits: public Domain, via Wikimedia Commons |
Finally, we can return to our young couple. The title of the present article is simply a matter of perspectives. From our level, we can clearly see them kissing, totally lip locked and in a mutual embrace. They are touching. As previously mentioned, all material objects are made up of a large number of atoms, meticulously stacked like the below image. It is this stacking that gives them their characteristics. A [solid] brick is definitely made up of atoms which are themselves not solid for the matter and is self-evident from the above quantum-mechanical picture of the hydrogen atom. If we could shrink ourselves to atomic dimensions, as shown beautifully in the popular science show Cosmos- A Spacetime Odyssey, we would find that what appears to be a solid object is fuzzy (the probability cloud) at the atomic level. In fact, from the perspectives of atoms, all macroscopic objects behave quantum-mechanically, being associated with a probability wave of amplitudes. For those who find it hard to picture the quantum fuzziness, our bodies is made up of a very large number of the Bohr Atom Model type of things sometimes stacked one atop the other or side-by-side or diagonally or in any other orientations in a crystal lattice.
.png) |
| The crystal lattice of a simple cubic structure Image Credits: Author's Computer |
It is the sheer number of atoms that gives substances their characteristics - solid, liquid or gas. In a crystal lattice, atoms are arranged in a definite order and there is always some distance (of a few nanometers) between two adjacent atoms. We already know that like charges repel and unlike charges attract. So when two atoms come closer, the electrons (or electrons' probability cloud) surrounding both the nucleus repels the other due to electrostatic interaction. Because of the Coulombic repulsion, there is, and forever will be a small distance of separation between the array of atoms that make the boy and that which make the girl, between us and our chairs, between our hands and the coffee cup which we think we are holding. This is why no one of us can sit on a chair or stand at someplace for what we are concerned about.
.png) |
| A repulsive Coulomb interaction takes place between two electrons. As a result two electrons (under ordinary circumstances) will always push each other. Image Credits: Author's Computer |
In a sense, the act of touching someone/something is a straightaway illusion. All objects comprise of atoms at the root level, and in turn, all atoms exist as fuzzy probability clouds, while further, the atomic nuclei are enveloped by an electrostatic field of negatively charged electrons, wherein the field lines permeate throughout the universe. What we know as touch or the touchy feeling is nothing but an illusion created by our brain while it attempts to interpret the electrostatic repulsion that manifests between the atoms on our skin and the object we are touching, rather try to touch. The moment someone touches us or when we touch something or if we firmly grasp the coffee cup between our palms, at the atomic level, the array of atoms is getting closer. As they close in, the Coulombic repulsion becomes the dominating means of interaction and pushes both the atoms apart. The touch receptors underlining our skin can experience and detect this Coulombic interaction which our brain interprets as the sensation of touch.
As a concluding remark, it is worth mentioning that the word touch sets a false premise to the article. Touch is a human way of experiencing things. But atoms do not experience things that we do, they do not touch like our young couple, they interact as allowed by the four fundamental fields - electromagnetic, strong, weak and gravitation. From our perspectives, the things that we touch have a definite size, shape, orientation, and boundary, and they exist for the matter. So we can hold them with our bare hands. But atoms are small, they are not definite, and over and again, speaking of the bizarre implications of quantum mechanics, an electron might not exist unless we observe them. So we can not say that atoms touch. The act of touching is simply not applicable in case of an atom. Atoms only interact. What appears to be an act of kissing is merely a collection of atoms [of the boy] interacting with another collection [the girl] or considering the wavefunction aspect, the boy's wavefunction is interacting with the girl's, and finally if we think of the fundamental field interactions, then everything that we do, all that exists before us is nothing other than the field interactions. This is why we say we inhabit a deceptive universe.
 |
| It is a crazy universe. To sit is to hover. To touch is to push. Image Credits: Photo by pixabay |
Author's Note: I should say that through this article I have only presented an overly simplified explanation of what actually happens when we touch something. However, to paint a complete picture, I will rewrite this article exclusively in the language of quantum mechanics. When two atoms get close a lot of interesting things take place. Depending on the scenario, those two atoms can fuse or they can bond, maybe they would annihilate each other. To understand touch at the quantum level, we need to be at ease with the methods and implications of quantum mechanics, namely, the psi function, the Pauli Exclusion, Fermi statistics, Bose statistics, including Field Theory and the Standard model. However, to understand something hard, it is better to start somewhere simple. So for now, using only the things we are taught in school, we come to the conclusion that because of electrostatic interaction two atoms can never get close. Since we are ourselves made up of atoms, the atoms on the boy's skin repels the atoms of the girl and they are perpetually apart by a few nanometers. They will always be apart.
To Be Continued...


Comments
Post a Comment