MODERN PHYSICS AND THE OLD QUANTUM THEORY-BIRTH OF THE ATOMIC THEORY OF MATTER
Everything around us is matter, albeit ordinary matter, which in addition to energy forms the very basis of our objective reality. We, human beings, are also material bodies, and on the atomic scale, are not very different from a baseball bat. From the 1960s, cosmologists suggested that nearly 93% of our universe might be composed of a yet unexplained type of matter called dark matter and an equally mysterious form of energy called dark energy. Everything else, i.e., all the planets, moons, stars, and galaxies, are made up of ordinary matter. As of this date, we have not yet figured out what constitutes dark matter, just as a couple of hundred years ago, we were unable to figure out the basic constituents of ordinary matter. And it took a long time, starting from the great philosopher, Democritus, around 400 BCE Greece, until our Erwin Schrodinger, who finally gave a picture of the basic structure of matter in 1926. Such was the complexity!
Previous article- The Wave-Particle Duality of Light
Ancient Greece and India: Around 500 BCE, two Greek philosophers, Leucippus and his pupil Democritus thought our physical world was composed of tiny, indivisible particles called atoms, suspended in an infinite void. The modern word 'atoms' originated from the Greek adjective 'atomos' meaning, 'indivisible' or 'uncuttable'. The atomist theory or the philosophy of 'atomism' was mainly developed by Democritus. The atomists believed that if a substance body is continuously broken into bits and pieces, after a certain point, it would not divide further. According to Democritus, these ultimate building blocks of matter were too small to be seen with our eyes and they differed in size, shape, order and position. These atoms could move through the void and combine i.e., cluster in various ways to form the material things that we see around us. Depending upon their orientation and individual shapes, their macroscopic aggregate was either fluid like water or firm or solid like a rock. The atomists also believed that the whole universe consisted of an infinite number of atoms floating in an infinite void which combined to form the sun and the stars. Interestingly, atomism was also used to explain human sensations, feelings and perceptions, which was believed to be caused due to the different shapes and sizes of the atoms. It is also good to know that apart from the indivisibility of material bodies, the atomists thought about the indivisibility of space and time itself, and argued whether there was an absolute minimum unit of space and time.
Although Democritus has been credited for his atomic theory, around 350 BC, Plato, on the other hand, presented a different idea based upon the concepts of indivisibility. The latter believed that our physical world was composed of four basic entities-air, fire, water and earth, which were further composed of indivisible regular solids made up of congruent, regular and polygonal faces with the same number of faces meeting at every vertex. These geometrical shapes were further decomposed into numerous sets of scalene and isosceles triangles. Each of the aforementioned entities were associated with four types of platonic solids-earth was associated with the cube, or the hexahedron, air with the octahedron, water with the icosahedron and fire with the tetrahedron. Apart from these, there was a fifth platonic solid, the dodecahedron, which was associated with the whole cosmos to account for the heavenly bodies. According to Plato's philosophy, since the air was a collection of octahedrons, it was so smooth that humans could barely feel it. Water, on the other hand, was composed of the icosahedron, which had many sides and was approximated to a set of tiny balls which possessed the ability to slip away from the hand. As the platonic solids were made up of triangles, it was shown geometrically, that a set of triangles can combine to form another set, and as a result, it explained how some of the elements transformed into the other.
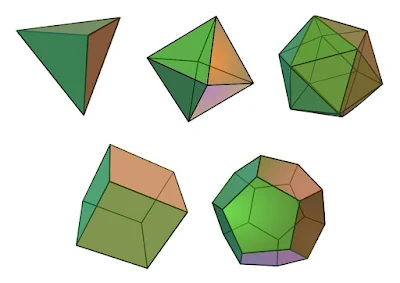 |
| The Five Platonic Solids. The Tetrahedron(fire), Octahedron(air) and the Dodecahedron(cosmos) are shown in the first row. The second row shows the Cube or the Hexahedron(earth), and the Icosahedron(water)/Image Credits: Максим Пе, via Wikimedia Commons, CC BY-SA 4.0 |
The atomic viewpoint was further extended by Aristotle, Epicurus, Lucretius, Diodorus Cronus and many other prominent Greek philosophers. It is to be noted that the philosophy of atomism was not restricted to the Greeks alone. In ancient India, during the Vedic era, i.e., around 800 BCE, the sage Aruni proposed that everyday objects were an aggregate of particles, too small to be seen with the human eye. These particles were known as 'kana', which were identical to the 'atoms'. In later years another philosopher, Kanada, proposed that everyday objects were made up of an indestructible unit, known as the 'paramanu'.
In due course, the atomist viewpoint was subject to many modifications and underwent a renaissance around the 17th century, which ultimately gave birth to the modern atomic theory. Galileo Galilei, Rene Descartes, Robert Boyle, Sir Isaac Newton and many others were all followers of atomism and 'corpuscularianism'. The latter school of thought suggested that matter was made up of tiny corpuscles, similar to the atoms, but were divisible. This corpuscular viewpoint was the basis of the practice of alchemy, and also, Newton's theory of light.
John Dalton: The modern atomic theory was the work of John Dalton. In 1803, while experimenting with various gases, he formulated the law of partial pressures, which states that the pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of the individual constituents. Before Dalton's atomic theory, in 1789, the French chemist Antoine Lavoisier put forward the law of conservation of mass, according to which the total mass before and after a chemical reaction remains constant. In 1799, another French chemist, Joseph Louis Proust, formulated the law of definite proportions. It says that a chemical compound is always made up of its constituent components in definite ratios and does not depend upon the compound's source or its method of preparation. Based upon these laws of chemical combination, Dalton gave the law of multiple proportions and used his atomic theory to explain how different elements combine to form various compounds. Sometimes known as Dalton's law, it states that the ratio in which a second element combines with the first element of fixed mass is always a ratio of simple, whole numbers. Based upon various experiments, one being that water could absorb carbon dioxide far better than nitrogen, he postulated the following:
- All elements are composed of extremely small indivisible particles called atoms.
- All atoms of the same element are identical and different elements have different types of atoms.
- Atoms can neither be created nor be destroyed.
- Atoms of different elements combine in simple whole-number ratios to form various compounds.
 |
| John Dalton. The father of modern atomic theory/Image Credits: Charles Turner, Public domain, via Wikimedia Commons |
Kinetic Theory and Brownian Motion: From the experimental study of the behaviour of a gas under varying conditions of temperature and pressure, physicists put forward a new set of gas laws, known as the kinetic theory of gas. According to the kinetic theory, a gas is composed of a large number of microscopic particles(atoms or molecules), which are in constant, random motion. The kinetic theory assumes that the pressure exerted by the sample of a gas upon the walls of the container in which it is enclosed is due to the random motion of numerous particles of extremely small magnitude which collide with each other, elastically, and with the walls of the container. These particles are Newtonian in nature and have definite kinetic energy and momentum. Their intermolecular forces of attraction and repulsion are considered negligible except during the time of actual collision between two particles. but since the kinetic theory was based upon the molecular behaviour of gasses, it was necessary to somehow prove the existence of these molecules or atoms.
In 1827, Robert Brown, a British botanist, made a very interesting observation. While observing a sample of pollen grains suspended in water under a microscope, he found that the grains constantly moved in a random, haphazard and zig-zag manner, without any predictability. It was further observed that in any colloidal solution, the suspended colloidal particles exhibit an eternal, irregular, to-and-fro motion, consisting of translations, and rotations. Each particle was observed to rise, sink, and spin chaotically. This phenomenon was termed Brownian Motion after Robert Brown, although he was neither the first to observe nor explain it. Almost eighty years after the discovery of Brownian motion, in 1905, Albert Einstein published a paper, explaining the phenomenon in light of molecular motions. Einstein proposed that the pollen grains or rather, in this case, the Brownian particles were moved due to the composite effect of the motions of a multitude of individual water molecules. This explanation of Brownian motion was also independently developed by Paul Langevin, Marian Smoluchowski, and experimentally verified by Jean Perrin, Theodore Svedberg, and others. The development of the Brownian theory was quite significant in the field of atomic and molecular physics, as it provided a definite proof of the existence of molecules, therefore validating the kinetic theory.
 |
| Sir J.J. Thomson/Image Credits: Public domain, via Wikimedia Commons |
Sir J.J. Thomson: Sir J.J. Thomson made a remarkable discovery while he was experimenting with the famous cathode rays inside a Crookes tube. The figure below gives a schematic diagram of Thomson's apparatus. One end of the evacuated glass tube, T, contained the negatively charged cathode, C, and next to that was the positively charged anode, A with a slit. Application of suitable potential difference between A and C produced the cathode rays that passed through the slit B, which was further connected to the earth and acted as a filter. The cathode rays emerging from B went through two aluminum plates connected to a battery source and was incident upon the glass surface at the round end of the tube. As the beam hit the screen it glowed due to phosphorescence. The dashed line indicates the undeflected beam in the absence of an electric field. If plate D was connected with the negative terminal of the battery, and E with the positive, the cathode beam deflected downwards. When D was connected to the positive terminal and E to the negative, the same rays deflected upwards.
 |
| Thomson's Apparatus/Image Credits: Author's Computer |
A Big Debate: In 1879, William Crookes observed that the cathode rays could be deflected under the action of a magnetic field, depending upon the polarity of the magnet. From this little observation, he concluded that the cathode rays were nothing other than some negatively charged particles or corpuscles. The luminescence at the round end of the tube did not depend upon the gas inside the tube or on the material of the electrodes. Therefore, it became evident that the cathode rays were a property of the electric field itself. Being influenced by Crookes, some physicists believed in the corpuscular nature of the cathode rays. But the German physicists, however, favoured Eugene Goldstein and advocated that the cathode rays were a disturbance of the aether, possessing wave-like characteristics similar to visible light. In 1892, Heinrich Hertz tried to observe the deflection of the cathode rays under the action of an electric field. Unfortunately, he failed to notice any sort of bending of the beam and thereby concluded that the cathode rays were unaffected in the presence of an electric field. This immediately implied that the cathode rays might not be a stream of negatively charged particles after all.
 |
| Crookes Tube with a Green Glow/Image Credits:D-Kuru, CC BY-SA 2.0 AT, via Wikimedia Commons |
In 1897, when J.J. Thomson designed an experiment to observe the afore-mentioned deflections, he concluded that Hertz's apparatus was faulty and the latter's vacuum was not high enough to show the deflection of the beam. Thomson's work was a culmination of all the previous attempts to discover the nature of the cathode rays. During that time, scientists believed that the atoms were indivisible, and the hydrogen atom formed the basis of all other higher elements. When Thomson measured the charge to mass ratio of the cathode rays, he found that they were 1800 times lighter than the hydrogen atom. Therefore, there had to be some material particle that carried the electric charge, was emitted from the inside of an atom itself and was necessarily smaller compared to the hydrogen atom.
This negatively charged particle, later renamed the 'electron' marked the discovery of the first sub-atomic particle. Soon, everyone realised that the electron was the ultimate carrier of electric charge, which finally settled a millennia-old mystery. But Thomson's discovery gave birth to another problem.
References:
- https://en.wikipedia.org/wiki/Atomism#
- https://www.britannica.com/biography/John-Dalton/Atomic-theory
- https://www.britannica.com/science/atom/Development-of-atomic-theory
- Gamow, George. Thirty Years That Shook Physics; The Story Of Quantum Theory. Dover Publications, Inc.
- Kaplan, Irving. Nuclear Physics. Addison-Wesley Longman Publications, Inc


Comments
Post a Comment