MODERN PHYSICS AND THE OLD QUANTUM THEORY: THOMSON'S PLUM-PUDDING MODEL AND RUTHERFORD'S NUCLEAR MODEL OF THE ATOM
The twentieth century, especially in its adolescent years, had witnessed a series of important discoveries, observations and revelations in the field of theoretical physics. Among them, the most striking was that an atom might be divisible, and in fact, be composed of a collection of minute subatomic particles, one of which was the electron. Quite naturally, it was necessary to obtain a clear picture of the atomic interior. Sir J.J. Thomson took this responsibility and presented to the world the first atom model, i.e., the Plum-Pudding Model, which appeared in a paper published in the Philosophical Magazine in March 1904.
Previous Article: The Birth Of The Atomic Theory Of Matter
The Plum-Pudding Model: While observing the deflection of the cathode rays under the action of an electric field, Thomson simultaneously measured the deflection due to the application of a magnetic field. From these observations, he firmly concluded that the cathode rays were a stream of negatively charged material particles or corpuscles emanating from the interior of the atoms themselves. It is good to note that Thomson's corpuscles were renamed as 'electrons' by G.J. Stoney to express the fundamental unit of electric charge.
Thomson thought that in the vicinity of the cathode, the intense electric field could split the gas molecules into subatomic corpuscles. If these corpuscles carry a negative charge, they would have behaved exactly like the cathode rays inside a typical Crookes tube. But an atom as a whole remained electrically neutral, i.e., they carried no net electric charge. So Thomson pictured an atomic model where an atom behaved like a positively charged sphere in which negatively charged electrons were embedded like raisins in a cake in a regular, ordered manner. Hence the nickname, the 'Plum-Pudding Model', was used by his contemporaries. The lowest energy state of the Thomson atom implied that all the electrons would remain fixed at their mean positions. But in an excited atom (i.e., when a small sample of gas is heated or ionised by applying strong electric discharge), the electrons would start to vibrate about their mean position. The classical electromagnetic theory states that an oscillating charge spontaneously emits electromagnetic waves. Thomson inferred that an excited atom would similarly radiate energy and account for the observed line spectra of the hydrogen atom.
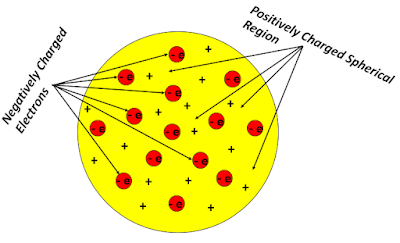 |
| The Plum-Pudding Model states that negative electrons are embedded uniformly inside a positively charged spherical shell/Image Credits: Author's Computer |
In the same year, a Japanese physicist Hantaro Nagaoka proposed an atomic model based upon the ring system of the planet Saturn. He pictured that the atom had a positively charged central region while the negative electrons revolved in fixed orbits.
Although Thomson's work was quite significant, within a few years it was found that the plum-pudding model was essentially flawed as it failed to explain the discrete nature or the line spectrum of hydrogen atom. During the latter half of the 19th century, the study of atomic spectra developed rapidly, which helped to determine the characteristics of various elements as they were excited to a higher energy state by applying a high voltage electric discharge.
Note: Right now, we should only be concerned about the line spectra of the hydrogen atom, which is seen as a series of narrow bright lines separated by dark intervals. The pattern of lines corresponding to definite wavelengths are given by the Rydberg-Ritz Formula and the series of spectral lines are grouped into the Lyman, Balmer, Paschen, Brackett, and Pfund series, all named after the scientists who discovered them.
 |
| Balmer Series in Hydrogen Spectra. The red line is the famous H-alpha line/Image Credits: Merikanto, Public domain, via Wikimedia Commons |
Rutherford's Gold-Foil Experiment: The historic 'Gold-foil Experiment' resulted from a series of experiments conducted by Ernest Rutherford, Hans Geiger and Ernest Marsden. Between 1908 and 1913, these physicists were trying to test the Thomson atomic model. According to the Thomson model, in which an atom's mass and charge remained uniformly distributed throughout itself, if an alpha particle(the nucleus of a doubly charged helium atom emanating from a radioactive source) were to collide with the atom, it would fly straight without undergoing significant deflection. As there are numerous atoms in a typical material substance, the alpha particles would suffer many small-angle deflections, and the resultant beam would be a diverging beam. Thomson's model also predicted that the electric fields inside an atom are weak enough to let the helium ion pass through without any high degree of deflection. Thus Rutherford's team set out to verify these predictions experimentally.
The following gives a schematic diagram of the apparatus used. A radioactive substance, viz., radon, placed inside a lead box fitted with a small opening, was used as a source for the alpha particles(A). The stream of particles was then collimated and made incident on a thin gold foil of thickness in the order of a few micrometres. A ductile and malleable material, such as gold, was chosen to be moulded into an extremely thin foil. However, in similar experiments, various other metals such as tin, mica, silver, copper, aluminium, etc., were used. The foil was so thin that most of the alpha particles passed without any significant deflection, and was detected(R) as a steady flash on the screen coated with a phosphorescent metal, i.e., Zinc Sulphide(ZnS). Some of the alpha particles were deflected from the gold foil and seen as scintillations on the ZnS screen. The number of scintillations and their angle of deflection was measured with the help of a movable detector.
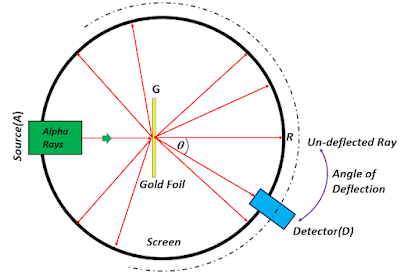 |
| The Gold-Foil Experiment performed by Rutherford and his team/Image Credits: Author's Computer |
Rutherford's team observed that most of the alpha particles passed straight through the foil, which further implied that the space inside an atom was mostly empty, as per the Thompson model. But it was simultaneously observed that some particles get deflected at extraordinarily large angles (greater than 90 degrees), and a small fraction almost deviated through 180 degrees! But calculations based upon the Thomson model suggested that the probability of scattering through angles more than 90 degrees was vanishingly small. Rutherford thought that this large-scale scattering further implied that the alpha particles suffered a stronger Coulomb interaction than what was predicted by the Thomson model. The electrons, which were almost 10,000 times lighter than the alpha particles, could in no reasonable way produce the observed effects. Experiments with various metal foil with a varying degree of thickness showed that the total number of alpha particles undergoing large-angle scattering was proportional to the number of atoms traversed by the alpha particle inside the metallic foil. It soon became a major crisis in physics, and following Rutherford's own words:
It was quite the incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you!
 |
| Ernest Rutherford/Image Credits: Public Domain, Wikimedia Commons |
Rutherford's Nuclear Atom Model: From a series of other experiments, Rutherford's team put together the 'Nuclear Atom Model'. Rutherford envisioned that the space inside an atom was mostly empty, and almost 99% of the atomic mass remained concentrated within a small volume. This small region, i.e., the 'nucleus', had to be positively charged to account for the large-angle scattering of the alpha particles. He also proposed that the negatively charged electrons revolve around the nucleus in circular orbits with very high speed. The stability of the atom was explained with the help of strong Coulombic interaction between the negatively charged electrons and the positively charged nucleus.
Thomson estimated that the atom could not be smaller than 10⁻¹⁰ meters. However, Rutherford's detailed calculations revealed the diameter of the atomic nucleus to be 10⁻¹⁴ meters and the atom as a whole was 10⁵ times larger than the nucleus. He also discovered that the mass of the nucleus was equal to that of the entire atom and the nuclear charge was given by Ze, where Z represented the number of electrons present in an atom circling and e was their nuclear charge. Z was determined experimentally and as a result, it became quite easier to calculate the number of electrons present in a typical atom of an element. Within a couple years, the proposed relationship between the atomic number of an element and its nuclear charge was identified by Antonius van den Broek, which was experimentally verified by Henry Moseley, who carefully determined the atomic numbers of a wide range of elements. The arrangement of the elements in the periodic table was therefore modified in accordance with Rutherford's theory, and the gaps were filled with the predictions of undiscovered elements.
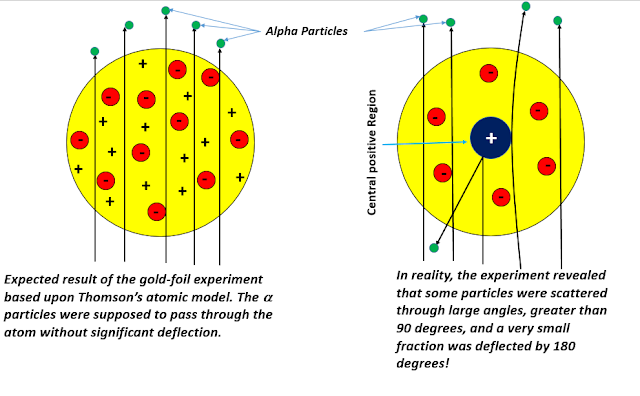 |
| Rutherford developed a new atomic model to explain the observations of his gold-foil experiment/Image Credits: Author's Computer |
Not Sufficient: The nuclear model of the atom was a highly successful feat, and there was little room for further questioning. But there was one big problem. Being based upon the classical treatment, Rutherford's model had a hard time explaining the stability of such an atom. At the centre of the atom was the atomic nucleus surrounded by a swarm of electrons, the number being different for each element. If it was assumed that the electrons in an atom are placed in stationary, fixed positions, then no mechanism would prevent the electrons from falling into the nucleus, owing to strong Coulombic attraction. If it happened, then the shell of electrons would collapse back towards the Thomson model of a sphere of positive charge, and the atom would become smaller, contrary to hundreds of experimental observations. To maintain stability, the electrons were pictured to revolve around the nucleus in fixed orbits, analogous to the model of our solar system. This again, gave rise to a far more complex problem. The solar system model implied that the moving electrons would be under constant acceleration, and from the principles of classical electrodynamics, all accelerating charged particles would radiate away their energy in the form of electromagnetic radiation. This continuous emission of energy would compel the electrons to lose all of their mechanical energy and thereby, spiral into the nucleus. Therefore, we would always have an atom rapidly collapsing itself into nuclear dimensions. It was further calculated that an atom of diameter 10⁻¹⁰ m, would collapse within 10⁻¹² seconds, i.e., one hundred millionth of a second, and the emission spectrum of hydrogen (shown above) would be a continuous one and not some discrete, spaced lines!
The problem of the emission spectra was ultimately resolved by Niels Bohr, who in turn, was a student of Thomson and Rutherford. Niels Bohr combined Rutherford's nuclear model with Max Planck's quantum hypothesis and created his very own Bohr Model of the atom. Bohr's treatment became the basis of what is now known as the Old Quantum Theory.
References:
- Eisberg, Robert and Resnick, Robert. Quantum Physics of atoms, molecules, solids, nuclei and particles, John Wiley and Sons, Inc.
- Gamow, George. Thirty Years That Shook Physics; The Story Of Quantum Theory. Dover Publications, Inc.
- Nobel Lectures Physics 1922-1941, Elsevier Publishing Company, Inc.


Comments
Post a Comment