MODERN PHYSICS AND THE OLD QUANTUM THEORY: BOHR MODEL OF THE ATOM AND SOMMERFELD'S MODIFICATIONS
Niels Hendrik David Bohr received the 1922 Nobel Prize in Physics for his outstanding contribution towards the development of the atomic model, known as the Bohr-Rutherford Model. Collaborating with Werner Heisenberg, Max Born, and Pascual Jordan, he put forward a new formulation of quantum mechanics, i.e., 'Matrix Mechanics'. His penetrating insights into the philosophy of quantum mechanics and the true nature of reality is known from the famous 'Copenhagen Interpretation'. Bohr predicted the existence of a new element, similar to zirconium, and was named 'Hafnium', after the Latin name for Copenhagen. It is interesting to know that in 1997, a synthetic element of the periodic table was named 'Bohrium' to honour the Danish physicist.
Previous Article: Thomson's Plum-Pudding Model and Rutherford's Nuclear Atom Model
Bohr Model of Hydrogenic Atom: In 1913, Bohr realized that the classical electromagnetic theory was not adequate to explain the stability of the Rutherford model. So he extended the quantum ideas of Planck and Einstein and put forward a theory for hydrogenic atoms. The term 'hydrogenic' applies to all one-electron atoms such as the ground state hydrogen atom, followed by the positively charged helium ion(He⁺), doubly charged lithium ion(Li⁺⁺), triply charged beryllium ion(Be⁺⁺⁺) and so on. The postulates of the Bohr model are as follows:
- The electron revolves around the nucleus, under the influence of Coulomb attraction, only in certain definite circular orbits without radiating any energy. These orbits are called stationary or quantised orbits of the atom and the electron is said to occupy a stationary state.
- The angular momentum(mvr) of the electron in a stationary orbit is an integral multiple of h/2π, i.e., 𝒎𝒗ₙ𝒓ₙ = nh/2π, n varies from 1,2,3,...., where m is the mass of the electron, 𝒗ₙ is its velocity in the nth state or orbit, 𝒓ₙ is the radius of the nth orbit and h is the Planck's constant. Once an electron is in its lowest orbit, it can no longer get closer to the nucleus.
- The electron can jump from one stationary orbit to another and release energy in the form of a photon if the jump happens from a higher orbit to a lower orbit. The frequency of the emitted photon will be given by 𝝂=(E₂ - E₁)/h, where E₂ and E₁ are the energies of the electron in the higher and lower orbits, respectively. The electron can also make a similar transition from a lower energy state to a higher energy state by absorbing a photon of the aforementioned frequency.
These postulates, particularly the second and the third, also known as Bohr's quantum condition and the frequency rule, provide a measure of the possible values of the orbital radii and also the energy of the electrons associated with each orbit. For simplicity of calculation, he considered the electron orbits to be circular, and assumed that the nucleus had infinite mass relative to the electrons. The correction of the finite nuclear mass and the possibility of elliptical orbits were applied much later.
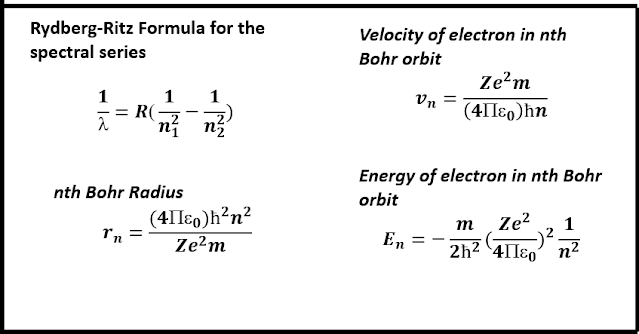
Subsequently, from detailed calculations, Bohr obtained a series of formulae for determining the radius of the nth orbit(also known as the Bohr orbit), the speed of the electron in this orbit, the energy associated with such an electron and also the frequency of radiation emitted during an orbital transition. He also discovered that the orbital radii and the orbital velocity depend on the n value, i.e., 𝒓ₙ was found proportional to n², and 𝒗ₙ was inversely proportional to n, which further implied, that the electron in the innermost orbit has the highest velocity. This n was later renamed the 'principal quantum number', which describe the electron's state. In the Bohr model, n = 1, 𝒓ₙ = 1 corresponds to the ground state of the hydrogen atom, i.e., the lowest possible energy state occupied by the electron. All other values of n indicate higher energy states. Bohr's equations were chosen in such a way so that the lowest possible energy state always correspond to n=1, unlike n=0 in Planck's quantum condition.
It is good to note that the first Bohr radius(the ground state) is 0.53 Å, where the electron velocity is 2.18x10^6m/s, and energy is -13.6eV. Thus, we get the concept of ionization energy, i.e., the minimum energy required to remove an electron from its ground state configuration. Again, the quantization of the angular momentum of the electron(2nd postulate) leads to the quantization of the total energy.
 |
| Some Important Formulae/Image Credits: Author's Computer |
Explanation of the Hydrogen Spectrum: To explain the emission spectrum of hydrogen, Bohr obtained a formula for the frequency of radiation emitted whenever an electron underwent a transition from a high energy level to a low energy level. His equation was similar to the experimentally determined Rydberg-Ritz formula and the Rydberg constant, which already appeared in the form of known fundamental constants.
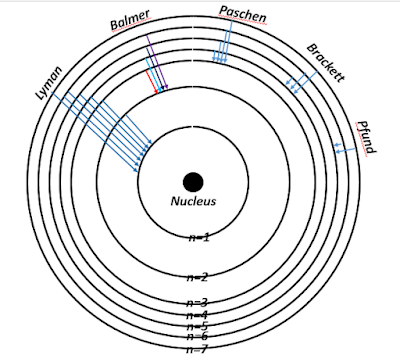
From the above formulae, it was observed that the values of λ, corresponding to n₁ = 1, n₂ = 2, 3, 4, ...,∞, and Z = 1, agreed with the experimentally observed wavelengths in the Lyman series. Thus, it was readily deduced that the Lyman series occur whenever an electron jumps from any of the higher orbits to the first orbit. Similarly, the Balmer lines occur when an electron jumps from any higher energy level or orbit to the second orbit, i.e., corresponding to n₁ = 2, n₂ = 3, 4, 5, ...,∞. The remaining set of spectral lines, viz., the Paschen, Brackett and Pfund series correspond to higher energy transitions.
Bohr's explanation of the hydrogen spectra or more precisely for a one-electron Bohr atom can be summarized as follows:
- When an atom is in its normal state, it means that the electron has the lowest possible energy, corresponding to n=1. This is its ground state or fundamental state(ground state originates from the German word 'grund', meaning fundamental).
- Under the action of an electric discharge or similar methods of excitation, an electron receives energy, and it must make a transition to a higher energy state or to an excited state where n is greater than 1.
- Since energy goes downhill, the excited atom will release all of its energy and return to the preferred normal state. The release of this excess energy will be accomplished by a series of transitions to subsequently lower energy states until the excited electron returns to its cherished ground state configuration. Every transition will be followed by the emission of electromagnetic radiation, whose wavelength will depend upon the initial and the final quantum numbers(i.e., initial and final value of n).
- A gas sample contains innumerable atoms and consequently, there will be various events of excitation and deexcitation processes; a measurement of its atomic spectra will reveal a multitude of spectral lines corresponding to all the possible transitions.
Bohr observed that his calculation of the Rydberg constant had a good agreement with the experimentally determined Rydberg constant. He also predicted the existence of the Lyman, Brackett and Pfund series, which were soon discovered via tedious experiments by the scientists after whom the respective spectral series have been named. The Bohr-Rutherford model, as it was called, worked well with the positively charged helium ion, for which Z=2. Apart from that, the absorption spectrum of one-electron atoms was easily understood with the help of the Bohr model. As it is not possible for an atomic electron to have total energy greater than the allowed energy for a particular stationary state, an atom can absorb only a discrete amount of energy from the incident electromagnetic radiation. Thus the process of absorption of energy becomes the exact opposite of the release of energy, and the lines of the absorption spectra will always have the same wavelength corresponding to the emission spectra.
 |
| Atomic transitions for the Lyman, Balmer, Paschen, Brackett and Pfund series/Image Credits: Author's Computer |
Limitations of the Bohr Model: There was no doubt regarding the validity of the Bohr model. However, not everything in this universe seems to be perfect and a scientific theory, no matter how profound it seems, often meets with some serious limitations and shortcomings. At first, the Bohr model did not apply to many-electron systems, and it could not be generalized to explain the spectra of elements like carbon, oxygen, neon, etc. Although, the model successfully predicted the spectra of ionized helium, but it failed to do the same for neutral helium. Secondly, it also failed to explain the differences in the intensities of the spectral lines. Third, spectrometers of high resolving power revealed that under the action of strong magnetic and electric fields, some spectral lines were composed of a group of closely spaced lines. This fine structure splitting of the spectral lines could not be explained on the basis of the Bohr model. Finally, the greatest flaw of the model was that it failed to provide any explanation behind the quantization of angular momentum of an electron moving in a circular orbit and Planck's quantization of the energy of a particle, such as an electron, executing simple harmonic oscillations. Finally, the Bohr model could not account for unbounded systems and scattering problems.
However, the major drawback of the Bohr model was the omission of the possibility of elliptical orbits of the electrons, although the latter was possible based upon the inverse-square Coulomb force.
Sommerfeld's Modifications: In 1916, William Wilson and Arnold Sommerfeld independently tried to provide a solution to the quantization problem in the Bohr model. Sommerfeld used these quantization rules to provide a more general picture of the atom, based upon elliptical orbits.
The Wilson-Sommerfeld quantization rule states that, for any physical system in which the coordinates are periodic functions of time, there exists a quantum condition for each coordinate. These quantum conditions are
In an attempt to explain the fine structure splitting of the hydrogen atom, Sommerfeld used these quantization rules and evaluated the size and shape of the allowed elliptical orbits, and also the total energy of an electron associated with a particular orbit. Therefore, he obtained the following conditions.
From the above, he in fact obtained two quantum numbers viz., the radial quantum number, and the azimuthal quantum number. The first equation gives the condition for quantized orbits and the second equation yields Bohr's second postulate i.e., the quantization of angular momentum. From which he further obtained that the energy expression given by Bohr hold for elliptic orbits and depends only on the principal quantum number, n. But for any value of n, there are multiple values of nᵣ and nᵩ. This is known as degeneracy. When nᵣ = 0, nᵩ = 0, the orbit of the electron becomes circular, therefore validating Bohr's concept of circular orbits.
Sommerfeld attempted to remove the degeneracy by applying relativistic corrections due to the mass variation of the moving electron. But, others soon realized that Sommerfeld's model was also flawed and it was necessary to have a more accurate description of the quantum realm.
Conclusion: The ''Old Quantum Theory'' is an umbrella term that incorporates all the previous attempts, starting from Planck's Quantum Hypothesis in 1900 up to the Bohr-Sommerfeld model of the atom in 1925. The quantum theory developed during this period is often expressed as the ''semi-classical approximation to the modern quantum mechanics''. The semi-classical approach is best explained by Bohr's ''Correspondence Principle'', which states that the quantum theory should agree with classical physics to the limit to which quantum effects become insignificant. The correspondence principle was used as a tool to explain the fine structure splitting of the hydrogen atom and inspired most of Bohr's work. From the above principle, he and Sommerfeld obtained a set of selection rules that would govern the type of transitions that an electron could make.
Within a very short time, Werner Heisenberg got influenced by the correspondence principle and formulated the famous ''Uncertainty Principle'' and a completely new branch of quantum mechanics known as Matrix Mechanics. In 1924, Louis de Broglie introduced the wave theory of matter, which was further extended by Albert Einstein. Finally, in 1926, Erwin Schrodinger put forward a wave equation or more precisely a ''Quantum Mechanical Wave-Equation'' and marked the beginning of ''Wave Mechanics'' and a probabilistic universe.
References:
- Eisberg, Robert and Resnick, Robert. Quantum Physics of atoms, molecules, solids, nuclei and particles, John Wiley and Sons, Inc.
- Gamow, George. Thirty Years That Shook Physics; The Story Of Quantum Theory. Dover Publications, Inc.
- Nobel Lectures Physics 1922-1941, Elsevier Publishing Company, Inc.

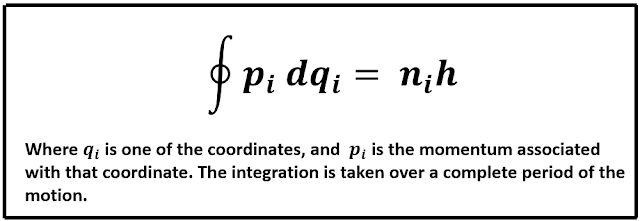
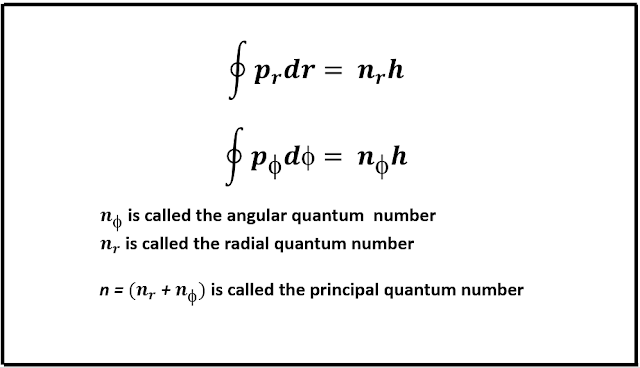


Comments
Post a Comment