HOW TO MEASURE THE CHARGE TO MASS RATIO OF AN ELECTRON-MILLIKAN'S OIL DROP EXPERIMENT
The German physicists, influenced by Eugene Goldstein, who is known for his works on the positive anode rays, believed that the cathode rays were purely a disturbance in the aether. J.J Thomson, however, demonstrated that the cathode rays were purely material in nature and were a stream of negatively charged particles that originated from the cathode. He also showed that these charged particles could also be deflected by the action of a magnetic field. Although Thomson's experiments cemented the fact that the cathode rays were indeed materialistic in nature, their true nature was a bigger question indeed. Were they atoms, or molecules, or something even fundamental? During those days, it was not possible to independently measure the charge or mass of a particle. So, he set out to measure their charge to mass ratio i.e., their specific charge. However, the greatest contribution was made by R.A Millikan who for the first time determined the charge of an electron and established the atomic nature of electricity.
Thomson's Method: Thomson's procedure for e/m determination is similar to the experimental setup already discussed in Birth Of The Atomic Theory Of Matter. A strong and uniform magnetic field was obtained by current flowing through a pair of Helmholtz coils on either side of the vacuum tube around D and E. Thomson adjusted the electric and the magnetic field to keep the beam undeflected and measured the velocity of the corpuscles ejected from the cathode. Second, he allowed the electric or the magnetic field to act individually and measured the amount of deflection. Combining the above results, Thomson arrived at a value of 1.7×10¹¹ C/kg for the electron. The specific charge(i.e., e/m) of an electron was observed to be independent of the nature of the cathode.
Before Thomson's experiments, the German physicist Arthur Schuster attempted to determine the e/m for the cathode rays. But his experimental methods produced a very crude estimate. Similar values of e/m were obtained from the experiments performed with radioactive emission of beta rays, thermionic emission from heated materials, and photoelectric emission. These helped establish the electron to be a fundamental constituent of matter.
At present, the accepted value of its specific charge is 1.758819×10¹¹ C/kg.
Dunnington's Method: In 1933, F.G Dunnington, developed a more sophisticated instrument to measure the e/m of an electron with the help of magnetic deflection. The image below shows a teltron tube, which is nothing more than a different type of a cathode ray tube and is used to determine the properties of electrons.
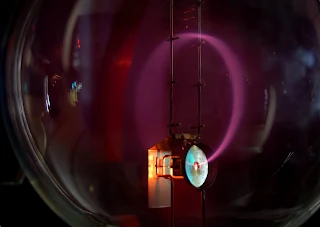 |
| A beam of electrons is deflected in a circular trajectory inside a Teltron tube/Image Credits: Marcin Białek, CC BY-SA 4.0 |
A stream of electrons are supplied from a heated metal filament and passes through a narrow aperture. A high-frequency crystal controlled oscillator supplies an alternating potential difference between the aperture and the source to accelerate the beam of electrons. The beam then passes through the aperture and emerges on the other side. The whole apparatus is evacuated to a high vacuum, and a steady magnetic field is applied. The electron beam bends under the action of the magnetic field and passes through the grid, and finally reaches the collector, which registers an electric current. The e/m ratio is given by the velocity of the electrons, the angle of deflection and the difference between the magnetic flux.
By this method, Dunnington obtained a value of 1.751×10¹¹ C/kg.
Millikan's Oil Drop Method: The electron is a fundamental constituent of ordinary matter, and its charge is also a fundamental constant of our universe. Although J.J Thomson succeeded in determining the charge to mass ratio, it was necessary to either know the charge or the mass of the electron. So Robert Andrew Millikan started a series of experiments from 1906 to measure the charge carried by a single electron. At first, he tried measuring the charge carried by tiny water droplets suspended via an electric field, but the experimental results were inaccurate. After much trial and error, he obtained a more precise value with the oil-drop apparatus.
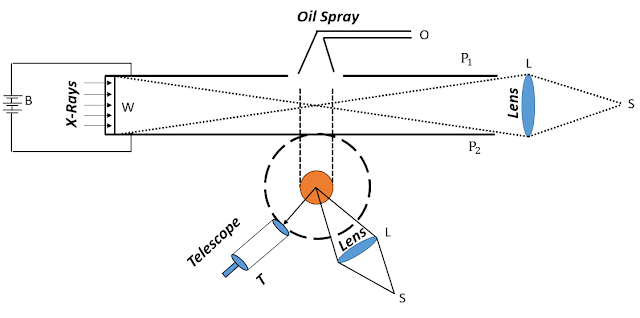
The above image shows the original apparatus devised by Millikan, and the over-simplified schematic diagram(below) depicts the working principle. Inside the cylinder, two parallel horizontal circular metal plates P₁ and P₂ were kept at a fixed distance apart, and separated by an insulation. On the top of the plate was a small hole through which fine droplets of oil were sprayed, via an atomizer jet O. A light beam was directed into the region between the plates from a source S and a telescope T was positioned, with its axis transverse to the light beam. The illuminated oil drops appeared like little stars when viewed through T, as the former fell and rose through the high electrical potential that was maintained between the parallel metal plates. The oil had to have low a vapour pressure. Otherwise, the fine mist of tiny droplets would have evaporated from the heat of the incident light used for illumination. That would have changed the mass of the droplets. But the experiment required the drops to maintain their size, shape and mass. The droplets acquired a charge as they exited the nozzle, and were further ionised by the incident x-ray through the window W.
In order to obtain a precise value of the electronic charge, Millikan took the following steps:
- The oil drops had to be homogeneous and spherical and maintain a constant density while falling through the potential difference(nearly 6000V/m) between the plates. The force acting on a particular oil-drop had to be measured by the momentum imparted to it, i.e., measure its speed, which must be constant throughout its passage. This would be impossible if the drop failed to maintain its shape and density. Hence, the diameter of the droplet was about a thousand of a millimetre, blown out of the atomizer and kept in a thermally isolated environment, to further reduce the effect due to conduction currents. Again, the droplets had to be large enough to neglect Brownian motion.
- To measure the force acting on a droplet, it was made to fall for a centimetre of the path through the electric field. The distance had to be large enough to track their fall. At first, they were allowed to fall freely between the plates and in the absence of an electric potential. The droplets fell with terminal velocity due to the air-resistance. The drag force and the terminal velocity were determined from Stokes' law.
- In the presence of the electric field, some drops were observed to rise, as the upward electric force was more than the downward gravitational force. Then a single drop was isolated, and the electric field was adjusted in such a way to keep the isolated drop suspended between the plates. Being suspended in air, it acquired some charge from the incoming X-rays.
Millikan's Conclusions: The oil-drop experiment was conducted over a significant number of drops and the value of the electronic charge was determined statistically. Millikan, who was aided by Harvey Fletcher, observed that the electronic charge acquired by the droplets was an integral multiple of a definite base value, i.e., multiples of 1.5924×10⁻¹⁹ C, which became the fundamental magnitude of the negative charge carried by a single electron. The charge was also found to be constant for all electrons.
Millikan's estimate was considerably higher than the currently accepted value of 1.602177×10⁻¹⁹ C. The error was due to a wrong estimate of the viscosity of air.
Significance: Millikan's experiment carried an immense significance as it established the fact that an atom is divisible, and the electron is a fundamental constituent of matter, having a definite charge and mass. It also established that electronic charge is a quantised quantity, and also a constant for all electrons. From the lecture he delivered at the Swedish Academy of Sciences while receiving his Nobel Prize, we learn from his own words, 'But the electron itself, which man has measured...., is neither an uncertainty nor a hypothesis. It is a new experimental fact that this generation in which we live has for the first time seen, but which anyone who will may henceforth see'.
Now, it was quite easy to calculate the mass of the electron, which was found to be nearly equal to 9.11×10⁻³¹ kg.
References:
- http://calteches.library.caltech.edu/4014/1/Millikan.pdf
- https://en.wikipedia.org/wiki/Robert_Andrews_Millikan
- Nobel Lectures Physics 1922-1941, Elsevier Publishing Company, Inc.




Very interesting post!
ReplyDeleteThank you!
DeleteNp! :)
Delete