NATURE'S MIRACLES: CHEMILUMINESCENCE AND COLD-LIGHT
Nowadays, we have all sources of artificial lighting, starting from the good ol' matchstick and candles to the filament bulbs and LEDs. But, for the past one million years, fire served as the basis of lighting in the absence of the sun, moon and stars. However, Mother Nature is full of surprises. Men have observed since antiquity that there are some insects that flash in the dark, mushrooms that glow with a ghostly green tinge, and finally, fiery seas. These myriad sources of natural light, often originating from living organisms, come under the umbrella term, Luminescence.
What is meant by Luminescence: The kinetic theory of matter states that all material bodies are composed of an aggregate of tiny particles(atoms and molecules) in a state of constant motion. This random motion, consisting of rotation, translation, vibration and collision, manifests itself as the internal kinetic energy of the body. Any material body with a temperature above absolute zero would, therefore, radiate away its internal kinetic energy in the form of electromagnetic waves emitted through blackbody radiation. Therefore, the same object glows due to this constant emission of radiation which occurs in the far-infrared spectrum. As our eyes are sensitive only to radiations having a wavelength between 380 and 700 nm, most of the infrared glow remains undetected. With the aid of a thermal imaging camera, we would notice that everything around us, starting from doorknobs, pen caps, coffee cups, including our pet cat radiates in infrared and far-infrared. We human beings also radiate energy with a characteristic wavelength of 10-12 microns, which is too small to be detected with our eyes. Again, we all know that if a piece of iron is heated, it starts to glow with a faint dull red, then deep red and slightly orange, to bright orange and finally yellow to yellowish-white. This is known as incandescence, i.e., the emission of electromagnetic energy and visible light from a hot body kept at a temperature greater than its surroundings. Incandescence can be treated as a special case of thermal radiation.
 |
| Vibrant glow of some fluorescent dye when exposed to ultraviolet light(black light)/Image by Pixabay |
Interestingly, the emission of visible light does not always need to be a product of the thermal motion of molecules or a violent chemical decomposition reaction. Whenever visible light is emitted from a substance which is relatively cooler than the surroundings, it is called luminescence, or in other words, cold-light. Luminescence is different from incandescence in the sense that the former can occur in substances that are at room temperature or below, unlike the latter, which does not occur before the Draper point, i.e., 978K, beyond which all solid materials start to glow visibly. Luminescence does not result from the thermal motion of molecules, but due to various atomic or molecular excitations via chemical reactions, electrical energy, and mechanical stress. Luminescence can never occur without the input of external energy.
The term luminescence comes from the Latin root lumen, meaning light. It was first introduced as luminescenz by the German physicist Eilhard Wiedemann in 1888 to distinguish light emission that are not accompanied by the rise in temperature. Luminescence can be defined as the spontaneous emission of visible radiation from an electronically excited species(or vibrationally excited species) that are not in thermal equilibrium with their surroundings. Based on the type of excitation processes, luminescence can be categorized into chemiluminescence, photoluminescence, crystallo-luminescence, electro-luminescence, mechano-luminescence, radio-luminescence and thermo-luminescence. Chemiluminescence is further subdivided into bioluminescence, electrochemiluminescence, lyloluminescence and candoluminescence; mechanoluminescence into triboluminescence, fractoluminescence, piezoluminescence and sonoluminescence; photoluminescence into fluorescence and phosphorescence.
Chemiluminescence: As mentioned earlier, luminescence occurs due to the excitation of electron species in an atom or molecule. A chemical reaction between two or more reactant species results in the formation of product species, and in most cases, is followed by the release of heat(if the reaction is exothermic), absorption of heat(if the reaction is endothermic). But sometimes, especially in certain types of oxidation reactions, the product species involves a reactive intermediate, which is a short-lived, energised and highly-reactive molecule. The reactive intermediate readily decomposes into a much stable product species and releases the excess energy in the form of visible photons, ultraviolet and infrared. Chemiluminescent reactions do not release much heat, unlike ordinary oxidation reactions such as the combustion of hydrocarbons, i.e., petrol or diesel.
The following two examples are enough to give a general idea about chemiluminescence in the liquid, i.e., the solution phase. It is good to know that chemiluminescence was first observed from the glow of elemental phosphorus in air, and not from the oxidation of organic compounds(luminol).
 |
| Luminol reacts with hydrogen peroxide and gives off its characteristic blue glow/Image Credits: Tavo Romann, CC BY-SA 4.0, via Wikimedia Commons |
1) Luminol: Luminol is a pale-yellow crystalline solid which is soluble in most organic solvents and insoluble in water. It is synthesized by a two-step process, which starts with 3-nitropthalic acid, a double benzene-ring structure consisting of a nitrogen dioxide group, and two carboxyl groups. It is heated with hydrazine in presence of an organic solvent such as triethylene glycol, glycerol, etc. This is a type of dehydration reaction that removes the hydroxyl group and transforms the 3-nitropthalic acid into 3-nitropthalhydrazide. The nitro group of the 3-nitropthalhydrazide is reduced to 3-aminopthalhydrazide(or luminol) using sodium dithionite.
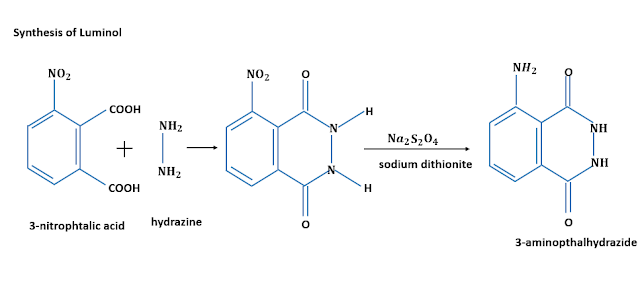 |
| Synthesis of Luminol/Image Credits: Author's Computer |
In presence of an oxidizing agent, such as hydrogen peroxide, and an activator like potassium ferricyanide, the hydroxide ions oxidize the NH group. The resulting cyclic peroxide is a highly unstable compound, and while it returns to a stable energy state, the excess energy is liberated in the form of visible light. However, the emission of light is a short lived phenomenon and it slowly fades away.
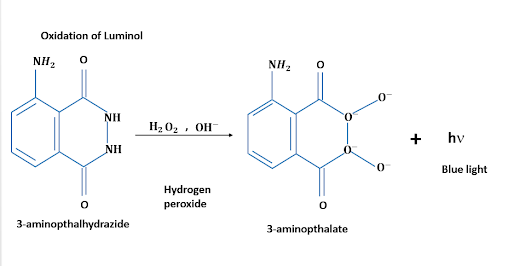 |
| Oxidation of Luminol/Image Credits: Author's Computer |
Luminol was first synthesized in Germany in 1902. In 1928, German chemist H.O. Albrecht observed that luminol(3-aminopthalhydrazide) gave an intense glow in the presence of blood, mixed with an alkaline solution of hydrogen peroxide. A few years later, in 1933, a German forensic scientist used luminol to detect traces of blood in a crime scene, and nowadays, this compound is widely used in the forensics. Hydrogen peroxide reacts with the iron blood, i.e., haemoglobin and produces oxygen ions. These in turn, oxidizes the luminol molecule and forms an unstable intermediate that readily decays into a stable compound, thereby releasing the excess energy in the form of a bright blue glow.
 |
| That beautiful glow/Image Source: David Muelheims (David Mülheims, Germany),CC BY-SA 2.5, via Wikimedia Commons |
2) Glow Sticks: An easily accessible and more popular way of demonstrating chemiluminescent reactions is the glow sticks. Glow sticks are typically used as decorations in music concerts, birthday parties, and can also be worn as a bracelet. However, it was designed as a signalling device for the military, particularly to be used underwater since they are water-resistant and can withstand pressures deep below the sea. Unfortunately, their inventor, Dr Edwin Chandross did not get much of a credit, and neither procure a patent for his invention, which later became a very important contribution in the field of chemistry. Based upon Chandross' work, several other inventors patent their glow sticks, and the chemical substance got its generic name, Cyalume in 1971.
 |
| Glowsticks used as a bracelet/Image Credits: By Pixabay |
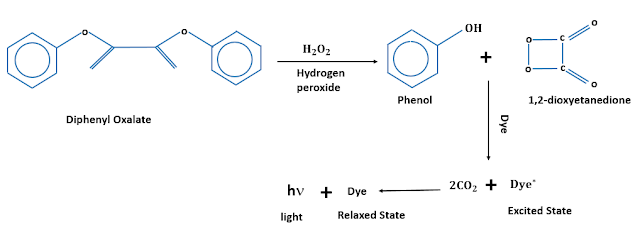
While working as a graduate student in MIT, Chandross was fascinated by the luminescence produced from the reaction of luminol. He was intrigued by the fact that a chemical reaction in general gives off heat, but not light. As a result, after acquiring a position at the Bell Labs, he wanted to test some of his own theories about the chemiluminescence of certain substances. Through trial and error with luminol, he discovered that peroxalate esters were the main ingredient behind chemiluminescence. Thus, after using a derivative of oxalic acid with hydrogen peroxide, the mixture lit up!
A typical glowstick contains a brittle glass container, enclosed by a flexible outer casing, preferably made out of plastic. The whole setup is designed in such a way that upon flexing, the inner container breaks, therefore releasing the necessary chemicals, which is then vigourously shaken to further catalyse the reaction. The outer casing holds a mixture of diphenyl oxalate, some fluoroscent dye and a suitable catalyst. The glass vial contains hydrogen peroxide. When the inner vial breaks, hydrogen peroxide mixes with the diphenyl oxalate ester, which in turn undergoes oxidation reaction and yields phenol and peroxyacid ester. The peroxyacid ester readily decomposes into carbon dioxide, and releases energy that excites the electrons of the fluoroscent dye. When the excited dye returns to its stable, relaxed state, it thereby releases a visible photon thereby, becoming chemiluminescent in the process. This is an exergonic reaction, which means it releases energy but not in the form of heat.
The colour of a glowstick depends upon the concentrations of the chemical used and of course on the type of dye, which is technically known as sensitizer or fluorophore Fluorophores are complex organic compounds that can absorb a light photon and re-emit from the de-excitation of the excited electrons. Glowsticks can be designed to emit red, yellow, yellow-green, orange, blue, violet, white, and sometimes, even infrared light. The intensity of emission can be adjusted by either using copious amounts of sodium salicylate, where the reaction proceeds much further, but the light is obtained for a short duration. Sometimes, heating the glowstick speeds up the reaction process, and thereby glow more brightly but for a short period. Conversely, lowering the temperature reduces the reaction rate, and the stick glows dimly for a long period. A glowstick can be manufactured to glow for a period of 5 minutes to 12 hours, and up to 24 hours under ideal conditions.
Different Types of Chemiluminescence:
- Electrochemiluminescence: Electrochemiluminescence or electrogenerated chemiluminesence is the emission of light as the result of an electrochemical process in a solvent.
- Lyoluminescence: The term Lyoluminescence was used by E. Wiedemann and G.C. Schmidt to describe the phenomenon of light emission when a solid substance is dissolved in a liquid solvent. Some substances, such as chlorides of sodium, lithium, potassium and even spices or milk powder, if heavily irradiated with ionizing gamma rays, and when dissolved in water, they start to luminesce.
- Candoluminescence: Candoluminesence refers to the phenomenon of light emission by certain substances heated to a particular temperature, which is exceedingly bright compared to the light emitted due to incandescence(i.e., blackbody spectrum). During the 19th century, before the advent of electrical lights, Welsbach gas mantle or limelight were used. It has been known for quite a some time that lime and similar oxides if heated to a high temperature would emit an intense light. From extensive research, it was revealed that the energy liberated during fuel combustion in a flame got stored in the combustion products. When these combustion products released their excess energy in the form of radiative transfer, an intense light was obtained, thereby making most oxides candoluminescent. Candoluminescence is also frequently known as the limelight effect.
- Bioluminescence: this deserves a separate article.
Conclusion: There are hundreds of other instances of chemiluminescent reactions that have been observed since the time of the discovery of phosphorus by Hennig Brandt in 1669. Brand experimented with animal urine, searching for the philosopher's stone, a mythical alchemic stone that could transmute ordinary metals into gold. Unknowingly, he stumbled upon a white, waxy paste that glowed in the dark and burned brilliantly. The new substance was named Phosphorous Mirabilis, or Phosphorus, meaning light-bringer. Another chemist, Johann Kunckel, moulded the substance into pea-like balls, enough of when placed in a glass of water yielded a soothing light and did not emit any heat. This was indeed one of nature's spectacular miracles, of obtaining light without a fire. It was further noticed that the substance gave off light in its solid phase, gaseous phase and also when dissolved in oil. Robert Boyle independently synthesized phosphorus and established some of the basic properties of luminescence. He concluded that bioluminescent light was cold light, and depended on the presence of air(oxygen). Luminescence was also observed in the reactions of strong acids and water with alkali metals such as sodium, potassium, ammonium hydroxide, etc.
References:
- https://en.wikipedia.org/wiki/Glow_stick
- https://www.britannica.com/science/luminescence
- Harvey, Edmund Newton. A History Of Luminescence; From The Earliest Times Until 1900. The American Philosophical Society, Philadelphia.
- Roda, Aldo. Chemiluminescence And Bioluminescence; Past, Present And Future. RSC Publishing


Comments
Post a Comment