MODERN PHYSICS AND THE OLD QUANTUM THEORY: PHOTOELECTRIC EFFECT AND THE IDEA OF PHOTONS
The discovery of the photoelectric effect had its roots in the painstaking attempts to detect the electromagnetic waves which were theoretically predicted by Maxwell. Maxwell's laws of electromagnetism provided a unification of the electric and magnetic fields, and henceforth implied that a variation of the magnetic field induced an electric field and vice versa. These oscillating electric and magnetic fields would generate electromagnetic waves propagating through free space at the speed of 186,000 miles per second. In due course, an exceptionally brilliant physicist, Heinrich Hertz, became the first to detect these invisible waves. At the same time, he also witnessed a weird phenomenon, which later became known as the famous photoelectric effect.
Previous Article- Blackbody Radiation and Planck's Quanta
Historical Background: Before the official discovery of the photoelectric effect, some physicists noticed a type of interaction between matter and radiation, in this case, solar radiation. In 1801, French physicist Alexandre-Edmond Becquerel, the father of Antoine Henri Becquerel, observed that ordinary platinum electrodes when coated with silver chloride or silver bromide and irradiated with visible light, produced an electric current. On the other hand, his contemporaries observed that certain metals would react to visible light and conduct electricity, thereby giving rise to the phenomena of photoconductivity.
However, the first observations of something similar to the photoelectric effect was reported by Heinrich Hertz while he tried to detect Maxwell's electromagnetic waves. Hertz designed an experiment in which at one end there was a primary spark gap where electric sparks were produced by the application of high voltage across a dipole resonator or antenna for simplicity. According to Maxwell's theory, the primary spark would generate electromagnetic waves which could be detected much farther from their source. Hence on the receiving end of his apparatus, Hertz placed another spark gap, where the secondary spark occurred simultaneously with the primary spark. This experiment gave a definite proof of the existence of electromagnetic waves. But, while investigating these waves, Hertz found it difficult to observe the faint sparks that appeared across the receiver. So he put it inside a black box. Interestingly, he observed that the maximum length of the sparks decreased when inside the box. At first he believed that the phenomenon was essentially electromagnetic. But after thorough investigation, it was found that a glass slab placed in between the primary and the secondary spark-gap could shield the secondary spark but a same piece of quartz could not. Upon breaking up the light from the primary spark into its respective components via a quartz prism, Hertz discovered that the secondary spark was more pronounced when exposed to light beyond the violet region aka ultraviolet light. Although he failed to thoroughly explain his observations, but his experiments laid the foundations of the photoelectric effect.
The next milestone was set in 1888 by Wilhelm Hallwachs when he devised a much simpler experiment to demonstrate the relationship between radiation and matter. In this experiment, he connected a zinc plate to a gold-leaf electroscope. The zinc plate was negatively charged which gave a negative charge to the leaves of the electroscope. Those leaves diverged away due to the repulsion of similar charges. Now, when the zinc plate was exposed to ultraviolet light, the leaves got discharged, and so did the zinc plate. Hallwachs figured out that the zinc plate emitted negatively charged particles under exposure to ultraviolet light. The phenomena intensified under the effects of oxidation, humidity and polishing of the zinc surface. It was further observed that this sort of emission only occurred if the frequency of incident radiation was more than a certain threshold limit, and did not depend upon the intensity of radiation. That's why no change was observed under visible light.
In the meantime, before the start of the 20th century, Sir J.J. Thomson, while investigating with cathode rays and ultraviolet light deduced that the cathode emitted material particles which he called corpuscles. These corpuscular particles were ejected whenever high-frequency radiation struck the metal surface and a current could be detected. These particles, renamed as electrons, were similar to those emitted from the ordinary cathode ray tubes.
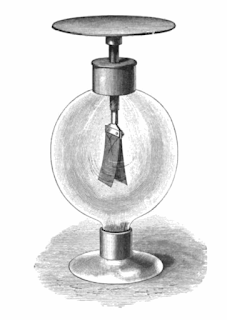 |
| The gold-leaf electroscope to demonstrate the above mentioned photoelectric effect/Image Credits: James Edward Henry Gordon, Public domain, via Wikimedia Commons |
Lenard's Experiment: These observations were soon followed by much detailed experimental analysis from Aleksandr Stoletov, Wilhelm Hallwachs and Philip Lenard. Stoletov discovered a direct relationship between the intensity of incident radiation and the photoelectric current, which also became the first law of photoelectric effect. On the other hand, Lenard, discovered a relationship between the energy of the emitted photoelectrons and the intensity of light. His experimental setup had a photosensitive emitter plate kept at a negative potential via a battery source. On the other end there was a collector plate, connected to an ammeter to measure the electric current. Both the plates were then enclosed within an evacuated vacuum tube and ultraviolet light was made incident upon the emitter plate. Immediately after illumination, the photosensitive material ejected negatively charged particles or electrons which raced towards the collector plate, generating an electric current through the ammeter and thereby completing the circuit.
If the emitter plate was kept at positive potential, so as to stop the electrons from reaching the collector plate, it was observed that some electrons still reached their destination. This meant the electrons were emitted with kinetic energy. However, a sufficient increase in the potential difference stopped the electron flow completely and the photoelectric current became zero. This stopping potential(V₀ or Vₛ), when multiplied with the charge of an electron gave the kinetic energy of the fastest moving photoelectron.
Experimentally, the stopping potential was found to be independent of the intensity of the incident radiation but depended upon the latter's frequency. Similarly, it was also observed that there was a minimum frequency, called the threshold frequency, below which no photoelectric emission could occur. The threshold frequency, however differed from material to material.
 |
| Philipp Eduard Anton von Lenard Image Credits: Wikimedia Commons |
Photoemission Characteristics: The following are the laws of photoelectric emission, which were first observed by Lenard and later confirmed by other physicists:
- The photoelectric current, i.e., the number of photoelectrons emitted per second is proportional to the intensity of incident radiation but independent of its frequency.
- The kinetic energy, or the velocity of the emitted photoelectrons varies between zero and a definite maximum. The proportion of photoelectrons having a particular velocity is independent of the intensity of incident radiation. However, the maximum velocity depends linearly upon the frequency of incident radiation.
- For a particular metallic surface, the emission of photoelectrons occurs if and only if the frequency of incident radiation is greater than the threshold frequency for that material.
- Finally, the emission takes place almost instantaneously, with negligible time-lag after the illumination of the emitter plate.
 |
| Lenard's apparatus to study the photoelectric effect/Image Source: Author's Computer |
Imminent Failure of the Classical explanation: The classical wave theory of light failed to explain the three major characteristics of the photoelectric emission. They are as follows:
- According to the wave theory, the amplitude of oscillating electric vector increases with the increase in the intensity of light. This further suggested an increase in the kinetic energy of the photoelectrons as the intensity of the beam increases. However experiments showed that the maximum kinetic energy of the ejected photoelectrons, Kₘₐₓ(equals eV₀) to be independent of light intensity.
- Secondly, according to the wave theory the photoelectric emission should occur for any colour(frequency) of light, provided that the beam is intense enough to impart sufficient kinetic energy to the electrons and dislodge them from their atoms. Again it was found that photoelectrons were ejected if and only if the frequency of the incident radiation was greater than that certain minimum frequency, i.e., the threshold frequency(𝜈₀). For frequencies less than 𝜈₀, the photoelectric effect did not occur, no matter however intense radiation was applied.
- Finally, if the incident light is of low intensity, meaning if it contains lesser energy, then the electrons would take a fairly good amount of time to absorb the energy, before being able to eject itself from its parent atom. But experimentally, it was observed, no matter what the intensity, even if it is far lower, photoelectric emissions were always instantaneous. There was no measurable time-lag between the illumination of the emitter plate and detection of the photoelectric current in the ammeter.
 |
| If the collector plate is given a sufficiently high positive potential, all the photoelectrons reach the plate and the photoelectric current saturates/Image Source: Author's Computer |
Einstein's Analysis and the Idea of Photons: Although Max Planck considered the quantization of material oscillators in the walls of the black-body cavity, he believed that electromagnetic radiation propagated like a continuous wave through free space. But Einstein reasoned that the incident electromagnetic energy itself behaved like a stream of tiny bundles, or packets of energy, also called quanta of energy. It is good to know that Einstein himself did not introduce the term photon, which was coined much later by the physicist G.N. Lewis.
According to Einstein's theory, when a single packet of energy collides with an electron in the metal surface, it is completely absorbed, thereby imparting all of its energy to the bound electron. This electron can now eject itself from its parent atom. Einstein introduced a term called the Work Function(W), which is the minimum energy required to remove an electron from the metal surface. Therefore, the maximum kinetic energy Eₘₐₓ and the corresponding maximum velocity of the ejected electrons are given by equation 1, known as Einstein's photoelectric equation. Next comes the threshold frequency(𝛎₀) which is the minimum frequency of incident radiation required for photoemission. This happens when all of the incident photon energy has been used up to liberate an electron from the metal surface such that there is no further energy left to provide the ejected electron its kinetic energy. Equation 3 could explain why Eₘₐₓ or previously, Kₘₐₓ was independent of the intensity of incident radiation. This is due to the fact that an increase in intensity means an increase in the number of photons striking the emitter, but not in the energy of the incident photon. Thus the number of photoelectrons emitted per unit time increases, but the energy of the photoelectrons remain the same. Secondly, for frequencies less than the threshold frequency, no photoelectric emission is possible. And finally, as the photoelectron emission is the result of a direct collision between an electron and a photon, there is no time lag.
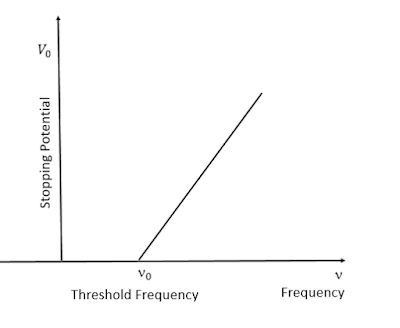 |
| Variation in stopping potential with frequency of incident radiation/Image source: Author's Computer |
The fourth equation gives the measure of stopping potential or the cut-off potential. If the collector plate in the photoelectric apparatus is kept at negative potential, the photoelectrons arising from the emitter plate are repelled back. For a certain value V₀ of this negative potential the most energetic electrons are turned back and the photoelectric current becomes zero. It is found that V₀ ∝ 𝝂 and the slope of the graph is given by h/e.
New Experiments: In order to disprove Einstein's idea of photons, Robert Millikan designed a very detailed experiment to measure Planck's constant. The experimental results were in complete agreement with Einstein's equation and Planck's Quantum Hypothesis. Therefore, Millikan's experiment opened up a new arena of particle physics and provided a solid proof in favour of the photons.
References:
- https://en.wikipedia.org/wiki/Photoelectric_effect
- https://en.wikipedia.org/wiki/Heinrich_Hertz
- https://www.britannica.com/science/photoelectric-effect
- Eisberg, Robert and Resnick, Robert. Quantum Physics of atoms, molecules, solids, nuclei and particles, John Wiley and Sons, Inc.
Next Article: The Existence of Photons




Comments
Post a Comment