MODERN PHYSICS AND THE OLD QUANTUM THEORY: BLACKBODY RADIATION AND PLANCK'S QUANTA
Modern Physics is a distinct branch of physics that originated in the early twentieth century, and includes all of the scientific developments up to recent times. Towards the end of the nineteenth century, some physicists observed that the Newtonian mechanics were only applicable to everyday objects having considerably large size, less velocity and equally lesser energy. But the laws failed miserably whenever the size of an object became smaller than 10⁻⁹m, or its velocity became comparable to 3x10⁸m/s and energy became very high. With the development of the atomic theory after J.J. Thomson's discovery of electron, and further failure of the classical attempt to explain the typical black-body curve, it was absolutely necessary to abolish the classical laws.
A Short Historical Background: During the mid-nineteenth century, there was a growing consensus among certain physicists that the laws of nature have all been figured out and physics were done. It was also believed that the classical laws i.e., the mechanical laws formulated by Newton, Lagrange, Hamilton, or the laws of electromagnetism given by Faraday and Maxwell, and finally the empirical laws of classical thermodynamics were adept at explaining the whole universe. But towards the end of that century, with the development of statistical thermodynamics, or the discovery of radioactivity and some associated phenomenon, the so-called fundamental laws of classical mechanics were brought under scrutiny. Albert Einstein brought a serious blow to the Newtonian physics when he abolished the traditional notion of space, time, and gravity, thereby developing the Special Theory of Relativity in 1905 and the General Theory of Relativity in 1916.
It is quite obvious that our universe consists of two basic entities-matter(i.e., apples, elephants, mutton chops, beer cans, etc.) and energy(i.e., electromagnetic radiation such a visible light, radio waves, gamma rays, etc.). During the early years of the twentieth century, the leading physicists conducted hundreds of experiments as they delved deeper into the microscopic nature of matter, electromagnetic radiation and the interaction of matter and energy. No sooner, it was observed that the experimental results failed to follow the classical predictions. Therefore, in an attempt to explain the famous blackbody radiation, Max Planck introduced a new idea-the quantization of energy and put forward his Quantum Hypothesis.
Thermal Radiation: It is the characteristic property of every material object(an apple or hot charcoal) to emit electromagnetic radiation, which is nothing other than the emission of thermal energy due to the spontaneous and random molecular motions between the material particles. At absolute zero, a thermodynamic system attains its lowest possible energy state. But above absolute zero, every material body is bound to radiate away its internal energy in the form of thermal radiation, produced due to inter-molecular motions. The characteristics of thermal radiation depends on various factors, such as absorptivity, reflectivity and emissivity of a substance. From Kirchoffs laws it readily follows that a perfect absorber is also a perfect emitter of electromagnetic radiation. A body with zero reflectivity is termed a black body, while on the contrary, a body with zero absorptivity is called a white body. Therefore a blackbody absorbs almost all of the incident radiation. Whenever a blackbody starts to emit radiation, it is called ''blackbody radiation''.
Blackbody Radiation: A blackbody is an idealised, hypothetical concept. So for practical purposes, Kirchoff theoretically showed that an enclosure whose walls are impervious to any type of radiation and if maintained at a constant temperature behaves as a perfectly back body. The radiations emitted by it are total radiations which depends upon the temperature and is independent of the material of the enclosure. Any speck of matter placed within that enclosure will be in a steady state and after attaining the temperature of the enclosure will emit black radiation characteristic of that temperature. The nearest approximation to a black body is a cavity with blackened inner walls and a small hole. Any radiation entering the enclosure through the hole will suffer successive reflections and get completely absorbed in that process. There is very little chance of its coming out and the cavity is said to absorb like a black body. Similarly, when this enclosure is heated to a certain temperature T, it will emit radiation made up of numerous contributions emitted from the walls of the cavity. The outpouring radiation will have the characteristics of black body radiation.
 |
| The typical blackbody spectrum acquires a peak at a particular wavelength/Image Credits: Darth Kule, Public domain, via Wikimedia Commons |
To Explain the Blackbody spectrum: The energy radiated at a particular temperature by a blackbody was plotted against different wavelengths and the curve was found to be a continuous one with the intensity of radiation acquiring a peak at a particular wavelength. The above graph shows that the radiant energy(plotted along y-axis) has a maxima corresponding to a particular wavelength(plotted along x-axis) and is small for very short or long wavelengths. In an attempt to explain the shape of the curve, Wilhelm Wien obtained a formula(Wien's law) which relates the intensity, I(λ,T) of radiant energy as an inverse function of the fifth power of wavelength. Wien's law fitted the experimental curve at long wavelengths but failed for the shorter wavelengths, where the energy density went towards infinity. Then came the Rayleigh-Jeans law after Lord Rayleigh and James Jeans. The Rayleigh-Jeans law which was based upon classical thermodynamics and electrodynamics, considered the radiating body as a collection of a large number of charged particles performing linear simple harmonic oscillation. These oscillating charges could emit and absorb electromagnetic radiation. At thermal equilibrium, the energy density of the radiation inside the cavity will be equal to the energy density of the atomic oscillators in the walls of the cavity. Further, according to classical equipartition theorem, the average energy of an oscillator, U(𝜈,T) at a temperature T is kT, where k is Boltzmann constant. But there was a major problem. Although the law obeyed the experimental curve at longer wavelengths, but as the wavelength became shorter and shorter, meaning as frequency became higher and higher the energy density diverged away towards infinity! Theoretically, it was found that a cavity could emit electromagnetic radiation with an infinite energy density, whereas experimental results showed a finite density. This misbehaviour of the Rayleigh-Jeans Law at higher frequency was termed ''The Ultraviolet Catastrophe'', and it was realised that a completely different approach was required to explain the spectral distribution.
Planck's idea of quantization of energy: Max Planck was bold enough to put forward a radical, out of the box postulate, known as Planck's Quantum Hypothesis. It states that: The material oscillators(in the walls of the cavity) can have only discrete energy levels rather than a continuous range of energies as assumed in classical physics. If a particle is oscillating with a frequency 𝜈, its energy can take only the values, 𝛆ₙ=nh𝜈, n=0,1,2,... where h is a constant, later called the Planck's constant. The quantity h𝜈 is called a quantum of energy. This implies that the particle can emit or absorb energy, not continuously in arbitrarily small amounts, but in multiples of quantum h𝜈. As the energy of the oscillators were fixed to be integral multiples of h𝜈, their energy was supposedly different from the classical expression of kT. From Planck's radiation law, the energy density of blackbody radiation was found to be as given below.
 |
| Planck's equations could perfectly explain the blackbody curve/Image Credits: From author's computer |
Planck's radiation law agreed very closely with the experimental curve and the value of Planck's constant was also chosen in a similar manner so that it agrees with the curve for all values of λ and T. It reduces to Wien's law as λ→0 and to the Rayleigh-jean's law as λ→∞.
Birth of A New Revolution: As mentioned earlier, Planck's radiation law agreed very closely with the spectral distribution curve of a black-body for all values of λ and T. Further, it also obeyed Wien's displacement law and Stefan's law, thereby providing solid evidence about the fundamentality of the quantum hypothesis. But Planck was reluctant to accept his proposal, and he believed that quantization of energy was strictly mathematical and in reality, that mechanism never occurred. Nevertheless, the idea of energy quanta helped physicists explain some of the most puzzling behaviour of matter and energy. In addition, the formulation of Planck's constant, whose value is 6.6260×10⁻³⁴m²kg/s, changed the way we view our world. Thus, he was awarded the 1918 Nobel Prize in physics, for giving birth to a new branch of physics.
Planck's constant is a fundamental constant in our universe, just like the speed of light, the fine structure constant and a few others. Life would not have appeared if its value been different than what it is. Without these physical constants, our universe could have been very much different, and it might not exist in the first place. However, that's another story.
References:


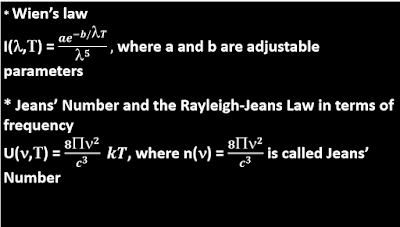


Comments
Post a Comment