MODERN PHYSICS AND THE OLD QUANTUM THEORY: THE EXISTENCE OF PHOTONS
Though it was pretty bold of Max Planck to propose that energy can assume discrete values instead of being continuous, nevertheless, he believed that electromagnetic energy could only propagate in a continuous pattern of waves. Albert Einstein modified his mentor's idea, therefore hypothesizing that not only energy was absorbed or released in quanta, but it also propagated in quanta. He also gave an explanation of the photoelectric effect based upon the hypothetical interaction of the light quanta with the metal's surface. Although Einstein's analysis perfectly matched the experimental results, it was absolutely necessary to detect the light quanta. From the individual experiments of R.A. Millikan and A.H. Compton, it was finally possible to detect the particle nature of radiation.
Previous Article- Photoelectric Effect and the Idea of Photons
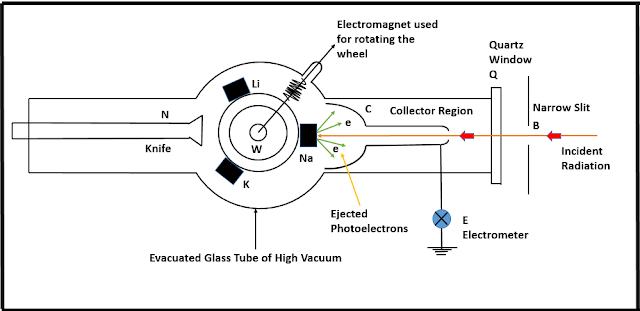
Millikan's Verification of Einstein's Claim: Nearly a decade after the publication of Einstein's paper, Robert Andrews Millikan published the results of the first ever direct measurement of Planck's Constant (h), and the work function (W). The following illustration gives a schematic diagram of Millikan's experimental setup. T represents an evacuated glass tube of high vacuum, Q is a quartz window, and B is a narrow slit through which light of high frequency enters the glass tube. This incident radiation falls on the surface of alkali metals-Sodium(Na), Potassium(K) and Lithium(Li), which are placed on a rotating wheel w. The wheel is rotated with the help of an electromagnet, and the knife N is used to shave off the surface of the metals so that every time a clean surface is exposed to the incident radiation. The ejected electrons from the metal surface are collected by a collector C, which is made up off strongly oxidised copper so that unnecessary electrons are not emitted from the metal surface when incident radiation from D passes through C. An electrometer E is connected to C for measuring the photocurrent. By applying a positive potential to w via E, a retarding field can be set up between w and C to stop the less energetic electrons reaching the collector.
Using radiations of different wavelengths, Millikan studied the photocurrent i for various retarding potentials V for each metal and also the nature of current by keeping the frequency/wavelength of the incident radiation fixed. The results he obtained are shown in fig (a), where I represents radiation of varying intensity. When the potential V of the collector C is made positive with respect to w, the photocurrent becomes constant i.e., we get the saturation current, which is obtained when all the electrons reach the collector. Quite interestingly, some amount of current flows even if V is zero, implying that the electrons are emitted with a finite velocity. When C is made negative relative to w, the current decreases and becomes zero at a particular value of v called the stopping potential or Vₛ, and is related to the maximum kinetic energy of emitted electrons as Vₛ=h(ν-ν₀/e), where e is the electronic charge. Therefore, Millikan was able to measure the maximum value of the kinetic energy of the ejected electrons. From the graph in fig (a) he also deduced that the stopping potential is independent of the intensity of incident light.
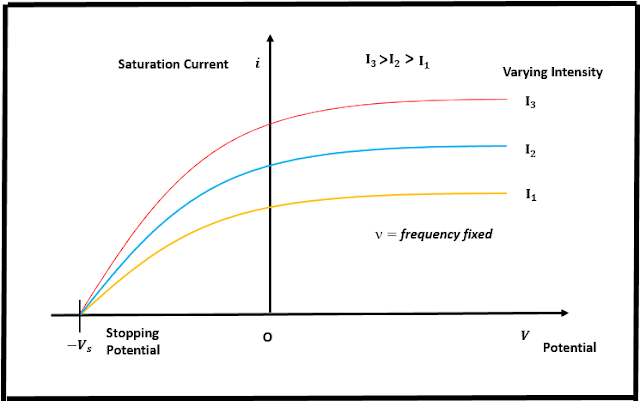 |
| Fig(a): Stopping Potential was found to be independent of the intensity of incident radiation/Image source: Author's Computer |
Further, Millikan tried to measure the variation of photocurrent with potential difference between C and D by using radiation of varying frequency but of same intensity. The saturation current was found to be the same as earlier, but the stopping potential recorded a higher value with increasing frequency. From fig (b) it can be seen that the saturation current is independent of frequency, but depends only on the intensity of incident radiation. Millikan repeatedly, measured Vₛ for a range of frequencies and for the three metal surface and a graph(given below) was obtained. The graph showed that the maximum kinetic energy of the ejected photoelectrons increases with ν, while the intercept gave the threshold frequency. The slope of the line was always the same, and the threshold frequency was found to be different for various metal surfaces.
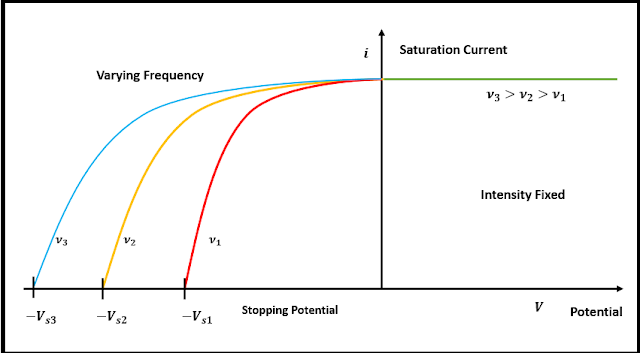 |
| Fig(b):Threshold frequency varied with the frequency of incident radiation/Image Source: Author's Computer |
Therefore, Millikan observed that his experimental results were a perfect match with Einstein's predictions, and the value of the Planck constant, determined in light of the photoelectric effect, was the same as the value obtained from the spectral distribution of blackbody radiation. This cemented the fact that the photons indeed exist, which was further proved by the experiments of Arthur Holly Compton in 1923.
Compton's Experiment: When electromagnetic radiation interacts with matter, an exchange of energy takes place between the atoms/electrons and the photon. There are three types of radiation-matter interaction. The first one being the photoelectric effect which is the low energy interaction between high frequency visible light to soft x-rays. Next comes the mid energy phenomenon i.e., the interaction of moderately energized X-rays that includes the Thomson scattering and Compton Scattering. At last, when photons of significantly higher energy i.e., hard X-rays and Gamma rays are incident upon an atom it gives rise to pair-production, photodisintegration and photofission.
Whenever a monochromatic beam of X-rays are incident upon an element with lower atomic mass, such as carbon, the incident beam is scattered at a different angle. Spectral analysis reveals the scattered spectra has two maximum intensities-one at the original wavelength and the other at a slightly different, but longer wavelength. According to the classical explanation i.e., considering the wave nature of light, the scattered X-ray should have a continuous spectrum. But experiments revealed the presence of an unmodified peak and a secondary peak, which was contrary to the classical picture. This shift in wavelength was found to depend only on the scattering angle, and was independent of the wavelength of the incident beam or the scattering material. This phenomenon is called the Compton Effect, after the name of it's discoverer Arthur Holly Compton.
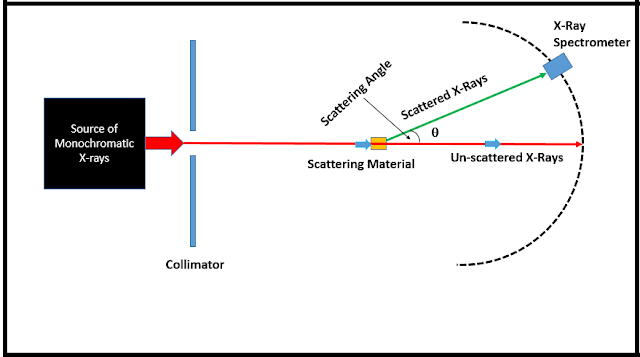
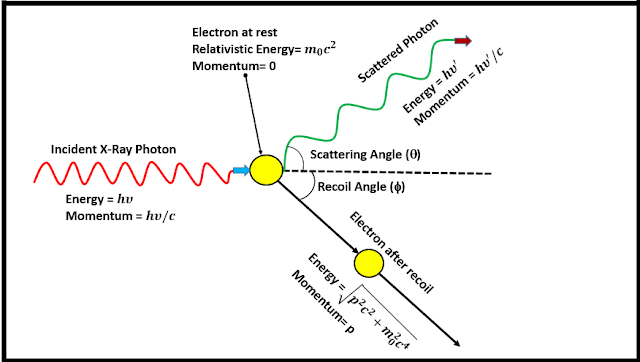
The above figure shows Compton's experimental setup, with the X-ray spectrometer being placed on a circular trajectory about the scattering material to detect the scattered X-ray at different angles and obtain their corresponding spectra. The following figure gives an idea of Compton's analysis, based upon Einstein's relativistic mechanics and quantum theory.
Compton's Explanation: Compton assumed, that the incident X-rays were a stream of photons having energy h𝜈 and momentum h𝜈/c, where 𝜈 is the frequency of incident radiation and c is the velocity of light. The collision between a photon and a free electron, initially at rest, is treated as an inelastic collision, where a part of the photon energy is transferred to the electron and it recoils. The photon gets scattered which now has a lesser energy and a lower frequency and hence a higher wavelength. Therefore he obtained the shift in wavelength(Δλ) as given below.
 |
| Wavelength Shift for various Scattering Angle/Image Source: Author's Computer |
This wavelength shift is independent of the wavelength or energy of the incident photons and depends only on the scattering angle. The quantity h/m₀c is known as the Compton Wavelength of the electron, which although a misnomer, gives the shift in the wavelength of a photon scattered off by an electron at an angle of 90° to the initial direction. The presence of the unmodified peak is due to the scattering of the photon by the atom as a whole.
And Everything Changed: Compton's experimental analysis was verified many times and it has been found that the recoil of the electron and the scattering of the photon occurs almost simultaneously. At that time, Compton's explanation was of great importance as it showed that electromagnetic radiation is indeed particle in nature and the photons are real particles with momentum, instead of being a fictitious entity used for the sake of easy mathematical analysis. It also established that the interaction of matter and energy is purely a quantum phenomenon.
References:
- https://en.wikipedia.org/wiki/Compton_scattering
- https://www.aps.org/programs/outreach/history/historicsites/millikan.cfm
- Eisberg, Robert and Resnick, Robert. Quantum Physics of atoms, molecules, solids, nuclei and particles, John Wiley and Sons, Inc.
Next Article: The Wave-Particle Duality of Light


Comments
Post a Comment