NATURE'S MIRACLES: PHOTOLUMINESCENCE, RADIOLUMINESCENCE AND THERMOLUMINESCENCE
On a literal note, phosphorescence means 'having a tendency to bear light'. Time and again, men have observed that certain minerals, when previously exposed to the sun, mysteriously acquired the ability to emit light even in darkness, and all by itself. Hence, every glowing entity of the old world, starting from minerals and rocks to bioluminescent mushrooms, marine planktons, and even jellyfish, were classified under the umbrella term phosphorescence. But true phosphorescence, and for that matter fluorescence - the two subtypes of photoluminescence, result from the interaction of certain solid(crystalline or amorphous) substances with visible light coming from the sun, ultraviolet, gamma- and X-ray radiations.
The Bolognian Phosphor: One of the most famous and the very first example of artificial phosphorescence is the Bolognian Phosphor, discovered in 1604, from Monte Paterno, near Bologna. While searching for the coveted Philosopher's Stone, Vincenzo Cascariolo, a shoemaker and an alchemist, stumbled upon a mineral of barium sulfate. After calcination, Cascariolo observed that the stone would spontaneously glow in the dark, as if it had acquired the light of the sun. The following is from E. Newton Harvey's book- A History of Luminescence.
The mineral was heavy spar, a native barium sulphate, rich in sulphur. Properly prepared, it would attract the ''golden light of the sun'' and seemed to be the appropriate material to convert ignoble materials into gold.....
The news of the discovery of the Bolognian stone spread like wildfire. Even though it failed to convert ordinary metals into gold, the ongoing search for luminescent minerals ultimately made mankind prosperous than ever. In the following years, a wide range of sulfur-containing minerals viz., fluorspar, zinc sulfide, calcium sulfate, strontium sulfide, limestone, Iceland spar, and many others were found to exhibit phosphorescence.
Peculiar Wood From Mexico: The earliest record of fluorescence dates back to 1565 when a Spanish physician named Nicolás Monardes used to treat kidney diseases with a special infusion of wood obtained from Mexico. This extremely rare specimen could turn ordinary water into beautiful shades of blue, and the resulting opalescence of the water changed with the angle of incident light. Modern research has revealed the blue opalescence came from the wood of two trees, Mexican Kidneywood(Lignum nephriticum) and the Narra tree (Pterocarpus indicus). During the 17th century, wooden cups made from the Narra tree were gifted to royalty, and the blueish water drunk from those cups were traditional diuretics. Today we know that the chemical substance responsible for this blue fluorescence is mataline, which comes from the oxidation of some of the tree's flavonoids.
Fluorescence And More Luminous Stones: Another important discovery that deserves mention is the discovery of luminescence in fluorspar, i.e., ordinary fluorite(CaF₂), in modern terminology. In 1852, G.G. Stokes, in his paper entitled ''On the change of refrangibility of light'', discussed the luminous effects produced by various samples such as quinine sulfate or fluorspar, being exposed to visible light. He observed that the transparent solution of quinine sulfate did not luminesce while placed in the visible section of the solar spectrum. But beyond the violet region, the solution started to glow with a blue light. As per the words of Stokes,
It was certainly a curious sight to see the tube instantaneously light up when plunged into the invisible rays: it was literally darkness visible. Altogether, the phenomenon had something of an unearthly appearance.
Through extensive research, Stokes concluded that the wavelength of the dispersed light was longer than the incident ultraviolet radiation. This is the famous Stokes Shift, by which certain materials can convert invisible UV radiations into visible wavelengths. Stokes introduced the term fluorescence, analogous to the luminous effects produced by fluorspar.
During the 1730s, Charles Francoise de C. Dufay catalogued numerous luminous gemstones displaying phosphorescence, fluorescence and even thermoluminescence, such as topaz(aluminium silicate), ruby, emerald, feldspar, aquamarine, olivine, tourmaline, sapphire, quartz, etc. He also observed that the colour of luminescence varied to a great extent. Luminescence in gemstones can sometimes result simply due to the reflection, refraction or transmission of light, and has nothing to do with phosphorescence or fluorescence.
 |
| A typical sample of opalescent glass appears to be blue from the outside, although a speck of orange light can also be seen. Image Credits: https://flic.kr/p/aYGa7 |
Most of the time fluorescence and phosphorescence was believed to be the same thing. And, Henri Becquerel, who accidentally discovered radioactivity through phosphorescence, found no practical distinction between the two. Since the discovery of the Bolognian stone, various attempts to arrive at a satisfactory conclusion have been made, with dozens of theories being published for almost 350 years and maybe more. However, the true mechanism could only be explained with the help of quantum mechanics, discovered in the last century.
Photoluminescence: Whenever electromagnetic energy interacts with matter, certain phenomena like photoelectric effect, Compton scattering, Rayleigh scattering, pair production, Cerenkov radiation, and of course, photoluminescence - phosphorescence and fluorescence, may be observed. As per Einstein's treatment of the photoelectric effect, electromagnetic energy travels in discrete packets called photons which carry a definite energy. This light-matter interaction can be further classified into three types: low-energy phenomenon i.e., photoelectric effect where photons lose their energy in liberating an electron from its parent atom, mid-energy phenomenon, i.e., Compton scattering where a photon gets scattered after suffering inelastic collision with an orbital electron, and finally, high-energy phenomenon like particle-antiparticle pair production.
Although similar in principle, photoluminescence is quite different from the photoelectric effect or Compton scattering, and the former occurs via photoexcitation, where an electron completely absorbs the incident photon's energy. Quantum mechanically, an electron can occupy several energy levels, called states. As the electron gains energy from the interaction with the incident photon, it is transferred to a higher energy state. Due to its tendency to return to the ground state, the electron loses some of its energy and liberates a photon of lesser energy and longer wavelength. The gif below, schematically shows an electron's excitation-de excitation mechanism. The blue arrow represents an incident photon, which strikes an electron and transmits to a higher energy level(excited state). When the electron returns to a stable state(least energy), it releases the excess energy in the form of a photon that has a longer wavelength. The orange arrow represents the emitted photon.
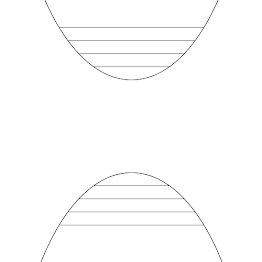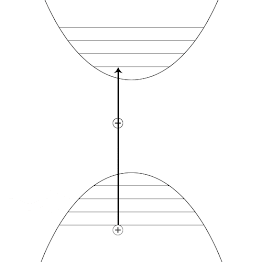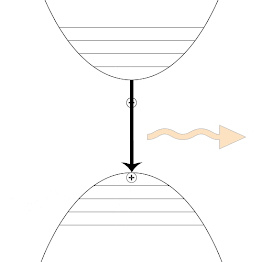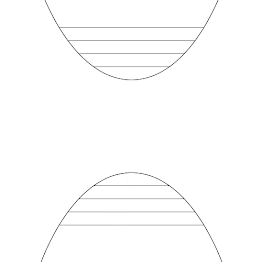 |
| The Jablonski diagram schematically shows the excitation-relaxation mechanism behind photoluminescence. Image Credits:BlyumJ, CC BY-SA 4.0,via Wikimedia Commons |
Based on the process of energy-loss and decay time, photoluminescence can be classified into fluorescence and phosphorescence.
- Fluorescence: Fluorescence refers to the emission of a longer wavelength(visible) light corresponding to lesser photon energy than incident radiation. When a fluorescent substance is irradiated with ultraviolet and other high energy radiation(invisible), generally UV light, the resulting light emission occurs almost instantaneously in the visible spectrum. Upon withdrawal of the radiation source, the emission stops instantaneously. A list of fluorescent substances include vitamin B2, quinine solution, highlighter ink(contains pyranine), anthracene dissolved in benzene, etc. In our daily lives, we have banknotes painted with an invisible fluorescent dye that glows under UV light and limits forgery. However, not all fluorites glow under UV. The presence of rare earth elements such as yttrium, terbium, europium, samarium and other similar activators gives them their fluorescing property.
- Phosphorescence: There is practically no difference between fluorescence and phosphorescence, except the former follows a relatively faster path while the latter goes through intermediate steps. Unlike fluorescence, a phosphorescent material does not glow instantaneously. It absorbs the incident radiation and re-emits over a longer period, and even after the removal of the radiation source. Some phosphorescent substances are europium compounds, zinc sulfide, strontium aluminate, and promethium.
 |
| The colour of light varies according to the fluoresceing substance Image Credits: Maxim Bilovitskiy, CC BY-SA 4.0, via Wikimedia Commons |
A phosphor is a substance that emits light in the visible wavelength after absorbing the incident electromagnetic energy from the photons. It is said to be fluorescent, if the phosphor immediately glows upon exposure to ultraviolet light, and phosphorescent if it glows in the dark after exposure to visible light. Interestingly, some marine and land organisms have been found to fluoresce, and desert scorpions glow green when exposed to UV light, i.e., blacklight in layman's terminology.
 |
| Some scorpions(dead or alive) have been found to emit a vibrant blue-green glow under UV light. Unfortunately, no one knows why or how they glow. Image Credits: Photo by form PxHere |
Radioluminescence: Strictly speaking, radioluminescence refers to the emission of visible light when photosensitive substances are exposed to a stream of high energy alpha-beta-gamma radiation originating directly from the decay of radioisotopes. However, it can also occur when energetic electrons or ions interact with the rarefied gas inside the cathode ray tubes and the glass at the anode end. Radioluminescence follows the same principle of electron excitation. As the electrons return to their ground state, the excess energy gets liberated in the form of visible photons.
- Cathodoluminescence: During the second half of the nineteenth century, William Crookes, among other physicists like Philipp Lenard, Heinrich Hertz, Julius Plucker, Eugen Goldstein, and Johann Hitorff, discovered various properties of the cathode rays(stream of electrons) which ultimately paved the way for J.J Thomson's discovery of the electron and the atomic structure. Crookes noticed that the high voltage electrical discharge under an equally high vacuum would cause the glass bulb at the anode end to glow with a greenish hue. As the high-velocity electrons collide with the glass surface, a portion of their kinetic energy gets transferred to the orbital electrons of the atoms in the glass, thereby exciting them to higher energy levels. To enhance the fluorescence, sometimes the end walls at the anode are coated with a thin layer of zinc sulfide.
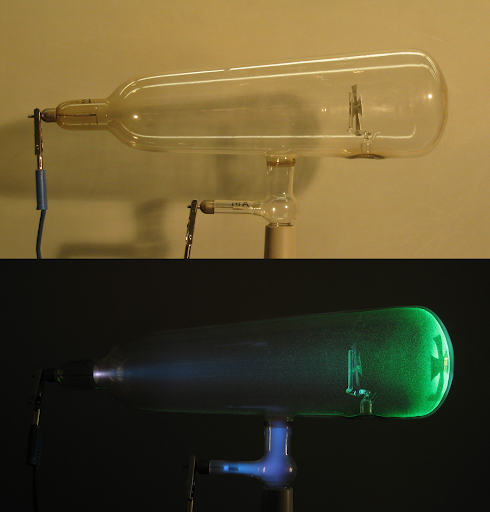 |
| The fluorescence at the anode end of the Crookes tube, with the Maltese cross, results due the collision of high velocity electrons and the glass. Image Credits: D-Kuru, CC BY-SA 2.0 AT, via Wikimedia Commons |
- Anodoluminescence: Eugen Goldstein, the discoverer of anode rays, i.e., a stream of positively charged ions emanating from the anode, observed similar luminescence. Even though Goldstein's discovery failed to motivate the scientific community, nevertheless, he continued his observations. From spectral analysis, he further revealed that cathodo- and anodoluminescence were separate phenomena. The spectra obtained after exposing lithium chloride to anode rays had the characteristic bright red line of lithium. However, when exposed to cathode rays, lithium chloride was found to be missing the red line. Similarly, soda glass emitted yellow luminescence under anode rays, and the characteristic green inside cathode tubes.
- True Radioluminescence: Radioactive elements spontaneously emit three types of radiations: alpha rays, i.e., the nuclei of helium atoms, beta rays, i.e., high energy electrons or positrons, and finally gamma rays, a stream of a very high energy photon. As a result, some radioactive substances can luminesce all by themselves, although the intensity will be significantly lower. True radioluminescence is obtained by bombarding a phosphor substance with radiation, such as the tritium tube(below). A tritium tube is a glass tube, the insides of which are coated with a phosphor and emit light by absorbing the electron's energy emitted from the beta decay of tritium.
 |
| A tritium tube Image Credits: CC BY 3.0, via Wikimedia Commons |
- X-rays: In 1895, Wilhelm Roentgen accidentally discovered a new type of invisible rays, and consequently another class of radioluminescence. While experimenting with cathode-ray tubes, he had the fluorescing portion covered with black paper to eliminate the green light from his experiment. Suddenly, he noticed a prominent green glow from the barium platinocyanide screen, even though kept at an appreciable distance away from the tube. This invisible ray, which he called X-ray, could travel through books, thin sheets of metal and even a human hand. Roentgen investigated these X-rays and discovered other radioluminescent substances such as calcium sulfide, uranium glass, Iceland spar and rock salt.
- Radium: In 1898, Marie Curie was fascinated to see the glow of her newly discovered elemental radium inside a dark room, and it's characteristic bluish glow made them analogous to stars of the night sky. Soon after this striking discovery, radium paint was used to manufacture glow-in-the-dark watch dials. Radium itself emits a very faint light, and is not visible without extremely dark conditions. When combined with a phosphorescent material such as zinc sulfide, high energy alpha-beta-gamma rays coming from the spontaneous radioactive decay, excites the phosphor substance which glows as shown below.
 |
| Dials of the above clock are glowing due to radioluminescence, which has previously been exposed to UV radiation. Image Credits: Arma95, CC BY-SA 3.0, via Wikimedia Commons |
Thermoluminescence: It has been found that certain crystalline substances do not become luminous even after absorbing electromagnetic energy from visible light, x-rays, gamma rays, cathode rays, and similar other sources of excitation. After irradiation, if these substances are warmed, they slowly start to emit light depending upon the rise and fall of temperature. It so happens that sometimes, excited electronic states gets trapped within the crystal lattice structure, which can only be freed with the application of heat energy resulting in thermoluminescence.
Author's Note: Photoluminescence can only be explained with the help of quantum mechanics, which is presently outside the scope of this article, and in no way I can simplify energy levels, or an electron's singlet-triplet state, into a bedtime story. I humbly accept my defeat.
- https://en.wikipedia.org/wiki/Luminous_gemstones
- https://www.edinst.com/blog/photoluminescence-differences/
- https://www.britannica.com/science/phosphorescence
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Spectroscopy/Electronic_Spectroscopy/Fluorescence_and_Phosphorescence
- https://www.thoughtco.com/do-radioactive-elements-glow-in-the-dark-608653



Comments
Post a Comment