To be in Love with the Setting Sun
Beginning on a personal note, I am not willing to accept that being a romantic means I have to get a girl, walk hand-in-hand and recite her a poem, not to mention a borrowed one. That is just part of being human. My true romanticism calls for spending endless hours watching the sun dip slowly below the distant horizon and sinking into the feeling of being a direct witness to the perpetual cycle of day turning into the night with the featureless blue sky gradually transforming into a vibrant and vivid canvas of scarlet red, brilliant yellow, bright orange and blushing pink.
A significant fraction of the general population believes that science is all about hard mathematical equations, endless rows of data, explosive chemicals, immortality pills and building exotic machines that will create a black hole and blow us back to the stone age. The worst of all, physicists and mathematicians are often portrayed as individuals long lost in thousand-plus pages of a book filled with weird symbols, little inverted triangles, a series of ridiculously italicised S (the integral) and something like Poseidon's trident (the psi function in quantum mechanics). Scientists are often not expected to understand art, have a taste for good music or literature, and more importantly, people reluctantly assume that scientists can not be romantics. However, people tend to forget that beauty lies in the eyes of the beholder. One person's appreciation for beauty does not necessarily need to conform to the other.
Take sunsets for example. Sunsets have been romanticized for generations upon generations through a numberless multitude of literary pieces and artwork, modern movies and music, and specifically, sunsets have become almost synonymous with young love. For some subtle reasons, sunsets appeal more to our inner senses than sunrises, even though both phenomena are like two faces of the same coin. Perhaps because of evolution and due to the fact that a million years ago, our distant ancestors walked across the open expanse of the African Savannah where the crimson red sun hanging low over the distant horizon served as their sole reminder to be back home before night falls, we have developed a special reverence for sunsets. A quick search on Google or any of the social media sites tells us that people have their own, unique reasons for liking sunsets. Young couples would mostly say that sunsets and the change of the sky's colour from a blander blue to the fiery shades of yellow and red give them a romantic feeling or something like that. Those with a knack for photography would probably blabber about white balance, natural lighting and how the intense glare of sunlight softens as the sun hangs low on the horizon, which is good for taking perfect photos. All in all, each according to their own, most people share a genuine love for sunsets.
 |
| Sunrise in the Masai Mara National Park Image Credits: flickr.com |
Sunsets are almost magical. However, very few people know of the intricate physics and equally abstruse mathematics involved in creating some of the colourful sunsets. All those crimson red hues, brilliant yellow and bright orange, with intermittent pinkish clouds merging into the deep dark shadow of the befalling night, do not happen at the flick of some heavenly fingers but manifest because of some specific reasons. On some days, the sun appears to be redder, sometimes the whole sky becomes pink, and occasionally, if there is a little rain in the late afternoon, then the eastern half of the sky turns a vivid blue with a sparkling rainbow while the low setting sun suddenly seems to be extra shiny. Also, sunsets seen across the open expanse of the tropical waters appear to be warmer and soul-touching than sunsets seen from densely populated cities such as New York or Kolkata (my home town). In fact, because of various physical and geographical factors and a host of other reasons, no two sunsets are the same, not even remotely. Every single day, it is a different sunset or a different sunrise.
Carl Sagan, the greatest communicator of science to the general public, in his book, the Pale Blue Dot - A Vision of the Human Future In Space gives a very apt description of the chemistry between science and romance. He writes, ''It is sometimes said that scientists are unromantic, that their passion to figure out robs the world of beauty and mystery. But is it not stirring to understand how the world actually works — that white light is made of colours, that colour is the way we perceive the wavelengths of light, that transparent air reflects light, that in so doing it discriminates among the waves, and that the sky is blue for the same reason that the sunset is red? It does no harm to the romance of the sunset to know a little bit about it''. I can not agree more, for being a straight third year physics undergrad, I have come to know Sagan's little bit, and I can assure you that instead of robbing me of my appreciation for beauty, it helps me see farther into the distance.
Who said being a romantic means I have to be mushy or lovey-dovey. As I have already mentioned in the beginning, my romance is in knowing that the sun never actually rises, much like it never sets, only our Earth is spinning about its axis from west to east which in turn, makes the sun appear to rise from the east and set in the west and rise again within a period of 23 hours, 56 minutes and roughly 4 seconds. Romance is thinking about how atmospheric refraction causes the bending of sunlight as it passes through Earth's atmosphere, as a result of which, the sun becomes visible above the distant horizon much before the upper limb of the solar disc geometrically rises above the line of sight looking straight towards the distant horizon. Romance is about contemplating the exact physical mechanisms by which the sun changes its appearance from a vermilion red dab at sunrise to a blindingly white disc during the day and returns to the former crimson red before it finally settles below the horizon.
 |
| A giantly giant nuclear reactor converting 600 million tons of hydrogen into helium and pure energy in every single second that ticks away Image Credits: flickr.com |
Every time I turn my eyes towards that vermillion red dab, all I see is a giant nuclear reactor, located some 150 million km from Earth, converting approximately 600 million tons of hydrogen nuclei into 596 tons of helium nuclei every second at a solar core temperature exceeding 15 million degreed Kelvin, while the remaining 4 million tons of nuclear material is readily transformed into pure energy as per Einstein's E=mc². I find it futile to comprehend the amount of energy liberated by the sun. But for my lay readers, to have a perspective of the power of the sun, let me tell you, each second the sun liberates the energy equivalent to 2 billion Tsar Bombas detonated all at once. In simple terms, the Sun powers itself by the nuclear fusion of four protons into a doubly charged helium ion, i.e., an alpha particle, accompanied by the creation of two positrons, two neutrinos and highly energetic gamma rays. Interestingly, due to thermal collisions and continuous absorption and reabsorption of the gamma photons, it takes more than 100,000 years for the gamma rays to finally come to the surface and escape into the surrounding space. But, by the time the gamma-ray photons from the interior of the sun reaches the photosphere, i.e., the visible surface of the sun, they lose a significant fraction of their initial energy, therefore decaying into ultraviolet, visible and visible photons rushing towards interstellar space at the speed of roughly 300,000 km/s. Technically speaking, the sun radiates energy across the electromagnetic spectrum extending from the low-frequency, long-wavelength radio waves carrying the least energy to the high-frequency, short-wavelength gamma radiations carrying the highest energy. However, because of the relentless absorption and reabsorption of the gamma ray photons inside the central core of the sun, much of the energy liberated from nuclear fusion stays within, while the sun radiates mostly in the ultraviolet, visible and infrared parts of the electromagnetic spectrum.
Maxwell's electromagnetic field equations imply that light propagates essentially in the form of a wave, manifest from the mutually oscillating electric and magnetic fields carrying electromagnetic energy through the vacuum of free space (source-free classical vacuum) exactly at the speed of 299,792.458 km/s. Unlike acoustic or water waves, light does not require a material media for propagation and unless there is some obstruction in its path (some charged particles, molecules, ions, brick wall, planet Earth, the moon, etc.), light will travel indefinitely and virtually speaking, for all eternity. Having said that, when travelling through a material media, viz., air, water, glass or oil, the electromagnetic waves interact with the constituent particles of the media and undergoes various types of light-matter interaction such as reflection, refraction, dispersion, absorption, scattering, and other kinds of interactions based upon the frequency of incident radiation and the characteristics of the travelling media. When sunlight (which is essentially white in appearance) passes through a glass prism, the incident beam splits into what we call the visible spectrum of light as seen by our visual system. Visible light encompasses particularly those wavelengths which carry sufficient energy, not more nor less, but the right frequencies necessary to stimulate, i.e., bring molecular changes to the photosensitive rod and cone cells at the back of our retinas. These molecular changes are registered by the brain, which after a series of complicated and if not unknown processes, give us the sensation of sight and our ability to marvel at those transcending sunset colours and that sparkling rainbow arching across the eastern hemisphere after a splash of fresh rain in the late afternoon.
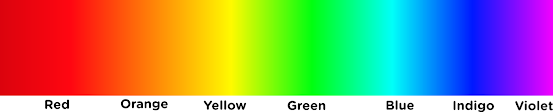 |
| The Visible spectrum of Sunlight consists of wavelengths extending from 750 nm on the red to 380 nm towards the extreme violet end. The remaining portion of the electromagnetic spectrum on either side is invisible to our eyes Image Credits: Wikimedia Commons |
The clear blue colour of the daytime sky and the warm crimson to yellow shades during sunrise/sunset come from a particular type of light-matter interaction called Rayleigh Scattering, named after its discoverer, the British physicist, Lord Rayleigh. Rayleigh scattering is the elastic scattering of visible light and other EM radiations by particles having dimensions much smaller than the wavelength of the radiation. Once more, EM radiations are nothing but mutually oscillating electric and magnetic fields carrying electro-magnetic energies. When these EM waves pass through Earth's atmosphere, the constituent molecules of air and the suspended aerosol particles (soot, pollen, dust, water vapour, etc.) get in the way of the free passage of light and act as scattering centres giving rise to secondary wavelets. The mutually oscillating electric and magnetic fields act on the negatively charged electrons and the positively charged nucleus of a typical gaseous atom/molecule, thereby forcing them to vibrate with a frequency similar to the frequency of the incident radiation. More accurately, Rayleigh scattering is the scattering of EM radiations by a harmonically bound electron. When the EM wave strikes a typical atom, a dipole moment is induced inside the atom due to the relative displacement of the negatively charged electrons (also called bound electrons, for they are tightly held together due to the Coulombic attraction forces) and the positively charged nucleus under the action of the electric field of the incident EM wave. These dipoles exhibit forced oscillations and themselves become centres of secondary radiations also known as scattered radiations.
Rayleigh scattering is a special case of the scattering of EM waves by harmonically bound electrons, where the frequency of the incident wave is much less than the natural frequency of the oscillating dipole. After suitable mathematical manipulation, one finds that the scattering cross-sections are proportional to the fourth power of the frequency of incident radiation or inversely proportional to the fourth power of its associated wavelength. In terms of individual molecules as Rayleigh scatterers, the intensity, I of scattered radiation is related to the incident wavelength, 𝝺 as follows:
From the above relation, we conclude that the shorter wavelengths (higher frequency) towards the blue and violet end of the spectrum will be more intensely scattered than the comparatively longer wavelengths (lower frequency) down from the green and yellow to the extreme red end of the spectrum. Sunlight or what seems to be white light is a mix-up of different wavelengths and corresponding frequencies ranging from 750 nm from the far red end to 380 nm towards the extreme violet end of the visible spectrum. Theoretically speaking, based on the above equation, the sky is supposed to look violet, for it has the shortest wavelength and is supposed to be more strongly scattered than blue light. Well, that is absolutely true. The sky in fact has a violet tinge. Unfortunately, our visual systems have evolved in such a way so that the peak sensitivity of the retinal cells corresponds to Red Green and Blue (RGB) portions of the visible spectrum, due to which we get an overall effect of seeing a blue sky, much like this picture I took from my balcony as I am typing this article. Even though the sky appears blue, it is undoubtedly violet.
Now that we have procured a rough idea of Rayleigh scattering we can finally come to our question of blue skies and red sunsets/sunrises. Earth's atmosphere is about 78% nitrogen and 21% oxygen followed by some other gases viz., hydrogen, argon, carbon dioxide, ozone, water vapour (which is not technically a gas; we will come to this later), neon, etc. The blue colouration of the daytime sky or the reddish sunset hues predominantly comes from sunlight interacting with the nitrogen and oxygen molecules which are almost a thousand times smaller than the wavelength of visible light. The moment a particular wavelength of light strikes an individual oxygen or nitrogen molecule, the oscillating electric field (mostly) induces a dipole moment between the positive and negative charges present inside the particular atom, which absorbs the energy carried by the incident wave and itself starts to vibrate with a frequency similar to that of the incident radiation. The molecule now acts as a radiating dipole and the secondary radiations emanating from such a dipole pertain to the characteristic colours of the sky.
On a clear sunny day, when the sun is at its highest point in the sky and if we were to directly stare into the illuminating solar disc we will notice that not only the sun is blindingly bright or that the surrounding sky in its vicinity has a whitish tinge instead of being blue according to the Rayleigh law, but the solar disc appears to have a yellowish tinge. The intense brightness of the sun comes from an altogether different type of scattering called Mie Scattering, named after it's discoverer Gustav Mie, for which we are going to need a separate article. Having said that, now if we turn our gaze in a direction away from the sun, but not to the horizon, we will notice the characteristic, deep blue sky, just like the above picture. Upon careful observation, we will also notice that the sky is not purely blue everywhere but fades off to a lighter and whiter shade near the distant horizon. This happens partly due to Mie scattering and some other phenomena associated with Rayleigh scattering which is beyond the scope of this article.
.png) |
| Figure 1 Image Credits: Author's Computer |
Rayleigh scattering by the individual molecules takes place uniformly in all directions. At high noon when we are looking directly at the sun, the sun's rays reach our eyes, following almost a perpendicular path through the earth's atmosphere. As it travels through the atmosphere, the incoming sunlight constantly collides with the constituent gas molecules, which efficiently scatter away the blue wavelengths from our direct line of sight, thereby giving the sun its yellow appearance. Additionally, from the final expression for Rayleigh scattering, we learn that the fraction of light scattered per unit length of travel depends on the number of molecules, i.e., scattering particles present in a unit volume of air. When the sun's rays are falling normally (perpendicularly), it suffers zero atmospheric refraction. Since a straight line path corresponds to the shortest distance between two points in space, during the daytime, when we look straight towards the sun, it appears yellow because all the blue wavelengths have been scattered from our line of sight, leaving behind only the yellow portion of the spectrum. Since red wavelengths are scattered the least, we see a yellow sun when it is at the zenith.
.png) |
| Figure 2 Image Credits: Author's Computer |
Now, as the blue light is scattered uniformly in all directions, the rest of the sky appears blue whenever we look away from the sun. This is evident from the above figure.
Owing to the curvature of the spherical earth and of the fact that it is constantly spinning about its axis, we readily understand that during sunset or sunrise, when the sun is low on the horizon, the incoming sunlight has to pass through an increased thickness of the atmosphere and reaches the observer tangentially, being further refracted by the dense lower atmospheric layers (figure one). When we are looking directly towards the setting sun, the light reaching our eyes has suffered multiple collisions with the gas molecules and has lost the entirety of its blue components, leaving behind only the longer wavelengths down from yellow to red. As the sun progressively nears the distant horizon, then following the Rayleigh law, it first takes on a deeper shade of yellow, then gradually shifts towards orange, until at the last few moments before it finally dips below the horizon, the incoming sunlight travels almost closer to the surface where it has suffered so many collisions with the gas molecules that the entire spectrum has gone extinct except the low-frequency red light.
The crimson red sun at sunrise or sunset is an extreme example of Rayleigh scattering by the gas molecules. Had there been no atmosphere, the incident sunlight would have faced no obstruction, and the sky would look completely black even when the sun rises high in the sky. There would be stars dotted across the black background of space with a perfectly white, brilliantly shining sun that would literally fry our retinas the moment we attempt to look at it.
As an ending note, I would like to say there is more to it than meets the eye. Sunsets involve a lot of physics which explains why not all sunsets are the same. On some days, it would look a brighter red, sometimes a dull red, maybe more yellow than red and on some other days, the sun might look like a squashed melon. And all of that can be explained by elementary physics. If you are reading this sentence, let me tell you, this is only the beginning, and the end is far from near.
.png)



Comments
Post a Comment