AN EVOLUTIONARY HISTORY OF ARTIFICIAL LIGHTING: THE AGE OF ELECTRIC LIGHTS [PART - 1]
The historical evolution of electric lighting is inseparably intertwined with the development of the subject of electricity, magnetism, and the field theory of the former two, known as electromagnetism. Gleefully, we are not at all concerned with Maxwell's equations or Biot's law or how electricity flows at the speed of light, but aim to trace out the path of our gradual transition from oil lamps to electric lights.
It All Started With Davy: Age-old investigations into the nature of electric phenomena eventually revealed their potential applications for lighting. It all started with a simple observation; the passage of electric current through a metallic wire (say copper) produces an appreciable quantity of heat (later identified as Joule heating). Further, if enough current is passed through the same metal wire, subject to an electrical resistance, the Joule heat intensifies into visible radiation. Using this concept, Sir Humphry Davy heated a thin platinum wire to incandescence by passing an electric current derived from a stack of 2000 voltaic cells (named after their inventor Alessandro Volta). But the resulting light was too feeble to serve any practical purpose, and the platinum wire, which consumed itself in the process, was concurrently expensive for single use.
In and around 1810, Davy demonstrated another source of electric lighting - the arc light. An electric arc is the visible manifestation of the sudden electrical breakdown of a gas (an electrical insulator under ordinary conditions), whence it allows the passage of an electric current. This sudden breakdown (discharge) ionises the air molecules, resulting in the formation of a plasma, and in turn, is seen as visible light. In the presence of a strong electrical potential difference between the two points of contact (in here the two carbon electrodes, shown below), a typical gaseous atom loses one of its electrons and becomes positively charged. In this way, there exists a sea of unbound negatively charged electrons that acts as carriers of electricity through the ionised gas.
Davy and other experimenters of electricity noticed that electrical sparks could jump across the small gap often left behind in a typical circuit. All he had to do was scale up the setup and produce a stable and sustained spark or, for that matter, an arc. Graphite being a good conductor of electricity, Davy connected a pair of carbon rods to opposite terminals of a voltaic pile and switched on the current. At the first attempt, the resulting arc was small, which he termed 'arch', and the reason is quite evident from the picture below. After some tinkering, he produced the brightest arc light at that time. However, it did not readily become widely accepted as it required thousands of voltaic cells (batteries), and the light was so powerful that the batteries drained themselves within a short time. Thus neither of Davy's inventions was of much practical importance to the general public.
 |
| The electric arch effect was also independently discovered by the Russian scientist by Vasily V. Petrov. The light is the visible manifestation of the spontaneous combination and recombination of ions and electrons. Image Credits: Achim Grochowski -- Achgro, CC BY 3.0, via Wikimedia Commons |
Arc Lamp: The principle of arc lighting is quite simple. In a typical arc light, two carbon rods attached to the opposite poles of an electric supply, known as the positive and the negative, are first brought into contact, and the current is allowed to pass. When the rods are slowly pulled apart, the visible spark gradually becomes brighter and brighter. At optimal separation, we can see the classic 'arch effect'. The temperature of a typical arc plasma can easily exceed 3000℃, with the positive electrode being at a higher temperature than the negative one. As a passing comment, it is worth mentioning that this temperature criterion separates the fourth state of ordinary matter - plasma, from our familiar, day-to-day solid, liquid and gaseous states. This intense heat readily vaporises the carbon rods, which in turn, gives off the characteristic white light. As the carbon rods are consumed, the spacing increases and the arc ultimately disappears within a minute or two. So to maintain the gap, hence the arc, the carbons rods had to be equipped with a complex feeding mechanism that kept them (rods) at the optimum separation, while the rods burned away at a constant rate.
Leaving behind all these engineering, arc lights were still impractical for public use, primarily due to their high operating temperatures and the emission of superheated carbon particles (buckyballs) posed a serious fire hazard. Apart from that, the light flickered and often went out violently. There was a constant hissing noise from the reaction between oxygen and superheated carbon followed by additional radio-frequency interference. Sometimes, the electrodes were enclosed within opalescent glass globes to reduce the light's intense glare, while a similar globe of quartz glass reduced the harmful UV radiations. Initially, the carbon rods did not last long more than an evening and had to be replaced every day. Since the latter was not much of a problem, arc lights were commissioned for public use, for it was significantly cheaper than gas or whale oil. Arc lights were mounted atop high towers and served as excellent communal lighting. The lights were so bright that ordinary folks imagined them to be miniature suns and moons capable of turning night into day.
 |
| The afterglow of a carbon arc lamp as it cools down Image Credits: https://flic.kr/p/Mv7X7 |
The first practical use of arc lighting was made possible by the Russian inventor Pavel Nikolayevich Yablochkov. In 1875, the streets of Paris and London were illuminated with Yablochkov's arc lights, also known as Yablochkov's candle - a fixture containing two carbon rods sandwiched by a layer of insulating material (ceramic). This whole thing was further enclosed in a glass globe to restrict the flow of oxygen, eventually slowing down the combustion of carbon and, in turn, increasing the light's overall lifetime. With the invention of better electrodes, i.e., copper-plated carbon electrodes or magnetite mixed with some titanium in definite proportions, along with sophisticated feeding mechanism, ac and dc dynamo to generate a stable source of electricity, arc lighting improved to a great extent. Some famous names associated with the industry include Leon Foucault, Charles F. Brush, Elihu Thomson, Edwin Houston, Thomas Alva Edison, William Wallace, Nikola Tesla and dozen others. Arc lighting was popular until the 1915s, after which it was replaced by gas-discharge lamps, metal halide, halogen and improved versions of incandescent lamps.
 |
| Different types of incandescent bulbs since its invention Image Credits: https://flic.kr/p/Mv7Sj |
Incandescent Light Bulb: Incandescent light bulbs follow directly from the principle of Joule heating, also known as resistive heating. It was first discovered and mathematically explained by James Prescott Joule in 1840 and later independently by Heinrich Lenz. The Joule-Lenz law states that the heat liberated by an electrical conductor is directly proportional to the product of the square of the applied current and the resistance of the conducting material. Electrical resistance is an intrinsic property by virtue of which all conductors (in fact, all material substances) resists the smooth flow of electricity. Because of this electrical resistance, more energy (work) is required for the smooth flow of electrons. This excess work manifests itself as heat (electrical energy transforms into thermal energy). Again, when a solid or a liquid substance is heated to temperatures above 525℃ (known as the Draper point) it starts to glow with a greyish, dull red. On further heating, the glow transitions to bright red, then orange, yellow, bright yellow, white, after which it either melts or sublimes. While the Draper point is nearly constant, the upper ceiling before sublimation is unique for all substances.
 |
| Carbon filament lamps. The bulb has blackened due to the oxidation of carbon. The filament has to be really thin to achieve the desired illuminating properties. Image Credits: Ulfbastel, CC BY-SA 3.0 ,via Wikimedia Commons |
But manufacturing that fragile wire (as seen in the above pictures), known as the filament, was not an easy task. As mentioned earlier, the amount of Joule heat liberated depends on the current and the resistance, which in turn, is further proportional to the length of the wire and inversely proportional to the area of cross-section. Hence the ideal filament would be a short and thin one, which would provide sufficient resistance to raise its temperature and glow in the visible spectrum. Further, the material of the filament must be able to withstand repeated cycles of heating and cooling. Unfortunately, seldom does theory meet expectations.
Following Davy's experiments, in 1841, Frederick de Moleyns received the first patent for a workable incandescent lamp with a platinum filament. Unfortunately, the light output diminished after some time as the bulb had already blackened out due to the rapid oxidation of the metal filament at high temperatures. The only way of eliminating this problem was to maintain a good vacuum inside the bulb. Unfortunately, producing an efficient vacuum was not possible until the invention of the Sprengel pump by Hermann Sprengel in 1865.
 |
| A tungsten filament bulb. The thin filament can be seen upon careful observation. Image Credits: Photo by form PxHere |
Incandescent bulbs became a commercial thing after the groundbreaking inventions of Thomas Alva Edison and Sir Joseph Swan. Around 1879, both of them independently created the most efficient filament material, i.e., carbon. While the former carbonised bamboo threads, the latter, applied the same technique to paper. Even though both were great innovators, Edison's design triumphed as it gave more illumination and was easy to manufacture, hence, cheaper. Their carbon filament bulbs continued to be in use until the discovery of a better filament material, called tantalum. In 1902, Werner von Bolton discovered that tantalum metal could operate at a higher temperature, meaning more light and was durable than carbon filaments. Within two years, Alexander Fredrich Just and Franjo Hanaman discovered tungsten to be a far better filament material than tantalum. Next, in 1908, William Coolidge's discovery of 'ductile tungsten' made the lightbulbs more luminous, durable, and long-lasting. Finally, in 1912 Irving Langmuir's breathtaking discovery that a mixture of inert (chemically non-reactive under ordinary conditions) gases such as nitrogen, argon, neon, and krypton, would greatly reduce the evaporation of the tungsten filament. He found that inert gases would deposit the evaporated tungsten atoms back onto the filament thereby, lengthening its lifetime.
Beyond The Incandescent Bulb: Even a few years ago, incandescent light bulbs were an indispensable thing. With the invention of energy-efficient and cost-effective LED lighting, most countries have slowly started to phase out the production of the old school filament bulbs. But there was a time when the whole of humanity witnessed in awe the witchcraft of electricity. A simple flick of a switch would turn night into day. In an attempt to come out of the literal 'Dark Ages', inventors across the world searched for the ultimate source of light, a light so bright that the sun would cast a shadow. So naturally, inventors went far beyond the incandescent bulbs, and it all got brighter and brighter. Here the following inventions deserve special mention.
Nernst Lamps: Before tungsten filament became popular, the Nernst lamps competed at par with the carbon filaments. The German national, Walther Nernst, was both a physicist and a chemist. While working in Gottingen in 1897, he developed the most advanced lighting system of that time. Instead of heating a metal filament in a partially evacuated glass bulb filled with noble gases, Nernst used a ceramic rod heated to incandescence. Unlike metal filaments, the ceramic rod, being a mixture of zirconium oxide, yttrium oxide, and erbium oxide, was immune to oxidation in air and bonus, the light was similar to sunlight.
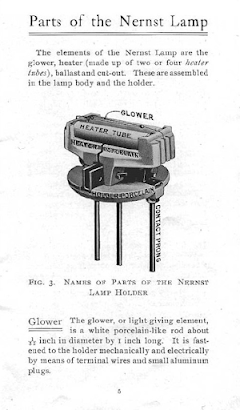 |
| Schematic diagram of a typical Nernst lamp with all of its necessary components Image Credits: Wikimedia Commons |
Also known as an incandescent glower, or Nernst glower, the Nernst lamps contain a set of heater tubes to be heated to red hot incandescence. This temperature rise would further heat the actual ceramic glower. The ceramic glower behaves as an insulator under ordinary conditions. But at elevated temperatures, its resistivity drops to a point where it allows the free flow of current and starts to glow brightly. The filament was enclosed in a glass bulb in order to isolate the hot element and reduce the risk of accidents. Although Nernst lamps had quite a few advantages over incandescent bulbs and arc lights, its major disadvantage was that the glower filament was not a conductor at room temperature. As a result, the need for a separate heater element made the lamps bulkier. But all in all, the Nernst lamp still remains a brilliant invention of the past century.
Halogen lamps: In an attempt to arrive at a better and long-lasting incandescent bulb, it was found that the tungsten filament would last longer if the bulb was filled with halogen gas. Thus the halogen bulb was developed jointly by Elmer Fridrich and Emmet Wiley from the General Electric Company, who received their patents by the 1960s. They discovered that glowing tungsten in contact with any of the halogen gases such as fluorine, chlorine, iodine, and bromine, would constitute what is known as the tungsten-halogen cycle. In an ordinary incandescent bulb, tungsten evaporates due to its high operating temperatures, resulting in bulb-blackening, even in a vacuum or a noble-gas filled atmosphere. But if halogen is kept under pressure, the evaporated tungsten reacts to form a halide, which does not deposit on the glass surface. When the halide reaches the hot filament, the tungsten gets separated and is redeposited on the filament. This greatly improves longevity while the bulb stays clear. The bulbs made from quartz or aluminosilicate glass can withstand high pressure in a small volume (for maintaining the halogen cycle) and a similarly high operating temperature, higher than ordinary incandescent bulbs.
 |
| Close-up view of the main halogen bulb-capsule. Image Credits: Modified from a photo by Stefan Wernli, CC BY-SA 2.5, via Wikimedia Commons |
Flashlights (Torch): Although the above lights were quite effective, it was natural to desire something portable. In fact, the whole incandescent lighting setup of a bulb and electric power supplied through central power stations had to be miniaturised. In this endeavour, the first dry cell battery was invented in 1897 by a German scientist named Carl Gassner. Simultaneously, tiny incandescent bulbs were manufactured which fit comfortably in a handheld cylindrical tube, fitted with a set of three batteries and equipped with a switch. In 1899, the English inventor, David Misell obtained the first patent for a flashlight - flash in the sense that they literally produced a brief 'flash' of light. These did not immediately become popular, primarily due to the little amount of light it produced and the use of crude batteries made the whole thing practically useless. Since the 1920s, as we slowly marched towards an electric society, people got more interested in buying flashlights due to their wide range of applications as emergency backup lights. Later in the late 90s, flashlights were equipped with LED, which has been the standard.
 |
| An early Philips flashbulb. The magnesium filament are the fibrous wires which immediately vapourise upon the passage of electricity, thereby emitting a strong flash. Image Credits: Thuringius, CC BY-SA 3.0, via Wikimedia Commons |
Flash (Photography): Although not directly linked with incandescent light bulbs or arc lights, photography flash or simply the flashbulb was a unique invention of the past century. The concept of flash was the brainchild of Robert Bunsen (the german chemist famous for inventing the Bunsen gas burner) and Henry Roscoe. While experimenting with magnesium powder, they found that the substance in contact with fire would explode violently, producing a light similar to natural sunlight (daylight). Its potential application to photography was realised by Edward Sonstadt, who with the inventor William Mather designed a type of magnesium ribbon which would burn inside a lamp. Another method was to ignite a small lump of magnesium powder and potassium chlorate (also known as Flash powder) on a tray. As igniting magnesium was particularly dangerous, due to its explosive properties, people preferred non-magnesium flashbulbs. Also known as the Sashalites (invented around 1930), these bulbs were made with thin foils of aluminium, magnesium, or zirconium, enclosed inside an oxygen-rich atmosphere within the bulb. When a current was passed, the metal immediately vaporised, giving a bright flash of light. Nowadays photography flashbulbs are completely different from the early versions. The modern ones are either LEDs or xenon-arc tubes.
Conclusion: Thus we see that even though electric lights were conceptualised as early as 1800, it was not much of a practical thing until 1875. As it always happens with a new invention, people were sceptical of trying out the new thing and were more than happy with open gas flames and oil lamps or tallow/paraffin candles. Towards the turn of the 20th century, Edison's invention of the bulb changed the face of humanity for good. In its length of time, electricity became cheaper and in all ways convenient than the smelly whale oil or exploding gas lamps. If we start our count from 1878, then within a period of a hundred years, i.e., by 1978, we have had all types of lamps, starting from incandescent, halogen, metal halide, gas discharge, fluorescent, induction, LEDs, and even laser; whence the latter might be the light of the future.
Sources:
- John, W. H. Men and Volts: The Story Of General Electric. Philadelphia, London, New York, J. B. Lippincott Company, 1941
- Bowers, Brian. ''Electricity'' An Encyclopedia Of The History Of Technology, edited by Ian McNeil, Routledge, London and New York, 1990
- Houston, Edwin J. and Kennelly, A. E. Electric Arc Lighting. Electrical World And Engineer, New York, 1902
- http://www.lamptech.co.uk/Documents/IN%20Operation.htm
- https://www.scott.k12.ky.us/userfiles/2505/Classes/37313/Resistance.pdf
- https://edisontechcenter.org/incandescent
- https://en.wikipedia.org/wiki/Timeline_of_lighting_technology
- https://en.wikipedia.org/wiki/Electricity
Continued.....


Very interesting read!
ReplyDelete